Figure 1.
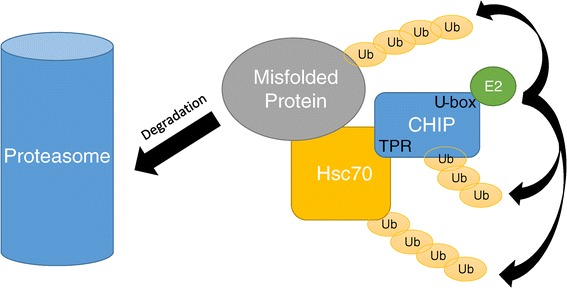
The dual role of CHIP as both a co-chaperone and an E3 ligase targeting misfolded proteins to proteasome degradation. CHIP binds to HSC70 by its TPR domain and bridges HSC70 to the misfolded protein. An E2 enzyme binds to the U-box domain and CHIP catalyses the ubiquitination reaction by attaching ubiquitin to the HSC70-client protein, targeting it to the proteasome. HSC70 and CHIP are also ubiquitinated, however this is not a signal for proteasomal degradation, but might play a role in their self-regulation.
