Figure 6.
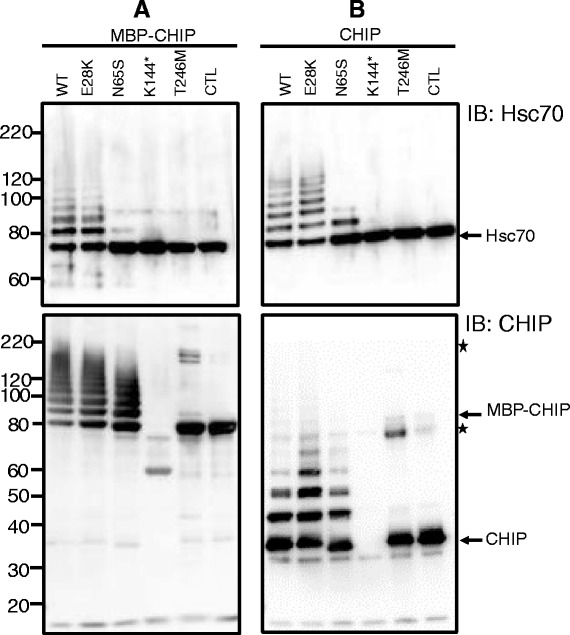
Different E3 ubiquitin ligase activity is observed for various CHIP mutants. In vitro ubiquitination was assessed using CHIP-WT and CHIP-mutant forms as E3 ligases and HSC70 recombinant protein as substrate for ubiquitination. Samples were analyzed by SDS-PAGE followed by immunoblotting using HSC70- and CHIP-specific antibodies. A reaction with WT-CHIP and without ubiquitin was used as a negative control (CTL). Both the levels of ubiquitination of HSC70 and auto-ubiquitination of CHIP itself was investigated using MBP-CHIP fusion protein (A) and tag-free (cleaved) CHIP (B). The lower molecular weight CHIP-K144* deletion mutant were detected as a MBP fusion protein, but not as a tag-free mutant, presumably due to reduced protein stability after removal of the MBP. The asterisks indicate CHIP forms mostly observed for CHIP-T246M and possibly representing protein dimers.
