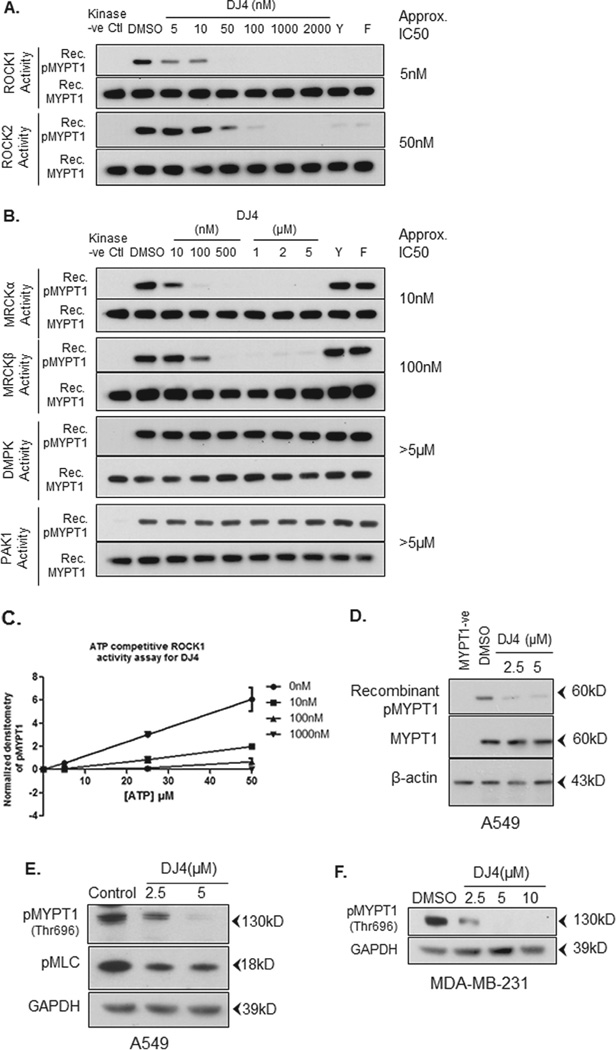
Fig. 3.
DJ4 selectively inhibits ROCK and MRCK over PAK1 or DMPK. (A and B) Recombinant proteins were incubated in the presence of MYPT1 peptide substrate, ATP (5 µM) and either DMSO or DJ4, Y27632 (Y) or hydroxyfasudil (F). Known ROCK inhibitors Y27632 and hydroxyfasudil (both at 1 µM) were used as positive controls. Samples without respective kinases were used as negative controls. Phosphorylation of the MYPT1 peptide substrate was detected by Western blot analysis using antibodies specific for phospho-Thr696 of MYPT1. Total MYPT1 (anti-MYPT1) was used as a loading control (C). DJ4 (10, 100 and 1000 nM) inhibits ROCK1 activity in an ATP competitive manner (5, 25, 50 µM ATP). (D) A549 cells were treated with DJ4 or DMSO for 24 h. Equal amounts of total protein lysates were incubated with recombinant MYPT1 peptide substrate in the presence of ATP (25 µM). Phosphorylation of the MYPT1 peptide substrate was detected by Western blot analysis using antibodies specific for phospho-Thr696 of MYPT1. Cell lysate without recombinant MYPT1 was used as negative control. (E) A549 cells were treated with either DMSO or the indicated concentration of DJ4 for 24 h. Endogenous phosphorylation of MYPT1 and MLC was detected using antibodies specific for phospho-Thr696 of MYPT1 and phospho-Ser19 of MLC. (F) MDA-MB-231 breast cancer cells were treated with indicated concentrations of DJ4 for 24 h and Western blot analysis was performed for endogenous pMYPT1(Thr696). GAPDH was used as a loading control.