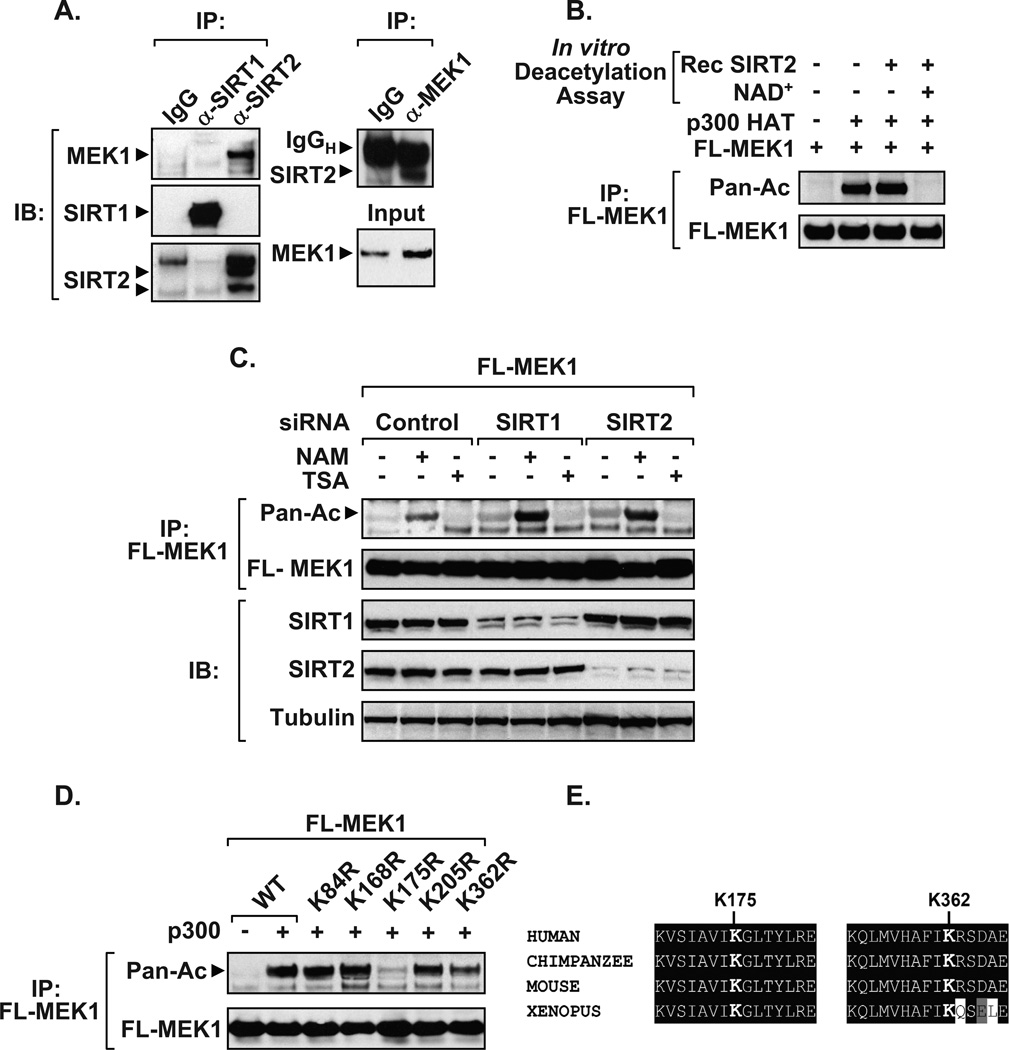
Figure 2. SIRT1 and SIRT2 deacetylate MEK1.
A) Endogenous interaction between SIRT2 and MEK1. Whole cell extracts from HEK 293T were immunoprecipitated using SIRT1, SIRT2, or rabbit IgG control antibodies and blotted using a MEK1 antibody (left panel). Re-probed blots show that SIRT1 and SIRT2 were effectively immunoprecipitated. Reverse immunoprecipitations confirm interaction between endogenous MEK1 and SIRT2 (right panel). B) Immunoprecipitated Flag-MEK1 is effectively deacetylated in vitro by recombinant SIRT2 enzyme in the presence of the NAD+ co-factor. Deacetylation assays were carried out as previously described (24). C) Knockdown of SIRT1 or SIRT2 potentiates MEK1 acetylation in response to NAM treatment. HEK 293T cells transfected with Flag-MEK1 and siRNAs were treated overnight with NAM or TSA and acetylation assays performed. Immunoblots performed on inputs confirm the knockdown of SIRT1 or SIRT2 compared to α-Tubulin. D) Lysine resides 175 and 362 are required for MEK1 acetylation. HEK 293T cells were co-transfected with plasmids encoding wild-type Flag-MEK1 (WT), or site-directed (K→R) mutants, along with vector control (−) or p300 (+). The acetylation status of immunoprecipitated epitope-tagged MEK1 was detected using an acetyl antibody, compared to immunoprecipitated Flag-MEK1 levels. E) ClustalW sequence alignment of MEK1 highlights the conservation of K175 and K362 across various species. Data presented in Figure 2 are representatives of at least three independent experiments. Small interfering RNAs to SIRT1, SIRT2, or control were purchased from Dharmacon. Additional antibodies used in Figure 2 include, α-Tubulin (Sigma), SIRT1 (Biomol), and SIRT2 (Abcam).