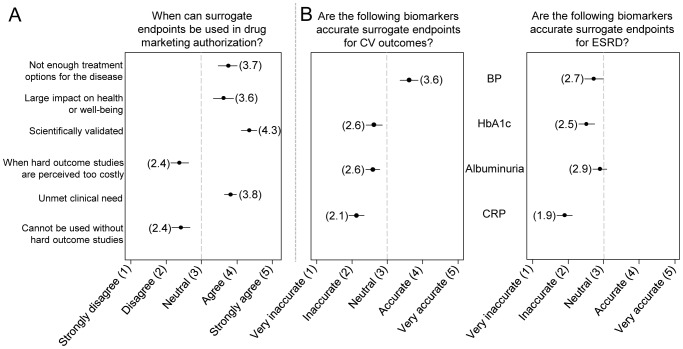
Figure 1. Pooled responses to survey questions.
A: pooled responses (mean+95% CI) of all stakeholders on when surrogate endpoints can be used in drug marketing authorization, provided that a post-marketing study with hard outcomes is conducted. B: pooled answers on which biomarkers are perceived as accurate surrogate endpoints for either cardiovascular outcomes or end-stage renal disease. Absolute mean values are provided in brackets. Abbreviations: CV, cardiovascular; ESRD, end-stage renal disease; BP, blood pressure; CRP, C-reactive protein.