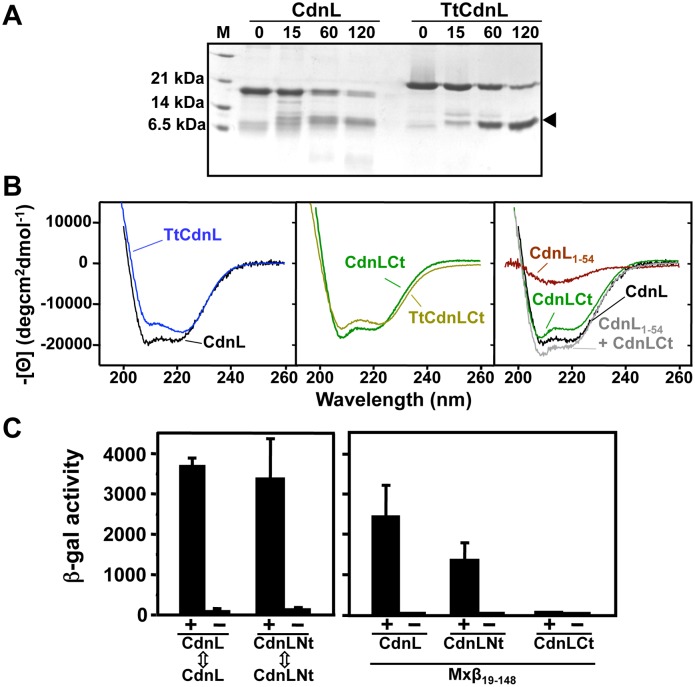
Figure 1. CdnL domain analysis.
(A) Limited proteolysis of CdnL and TtCdnL. Aliquots of the reaction mix (1∶100 w/w subtilisin:protein) at 30°C were withdrawn at the times (in min) indicated on top and analyzed in Coomassie-stained 15% SDS-PAGE gels (lane “M”: molecular weight markers). Arrowhead on the right points to the subtilisin-resistant fragment, which mass spectrometry and N-terminal sequencing identified as the ∼100-residue C-terminal region in both proteins. Note the slower mobility of TtCdnL, whose size (164 residues) is the same as CdnL. (B) Far UV-CD spectra of CdnL, TtCdnL, and their indicated fragments. (C) BACTH analysis of the interactions of CdnL and its domains showing reporter lacZ expression in E. coli transformed with plasmids pKT25 and pUT18 bearing cdnL or the gene for CdnLNt (left panel); or with a pUT18C construct of the gene for Mxβ19–148 and pKT25 with cdnL or its indicated fragments (right panel). pKT25 without insert was used in negative controls (“−”).