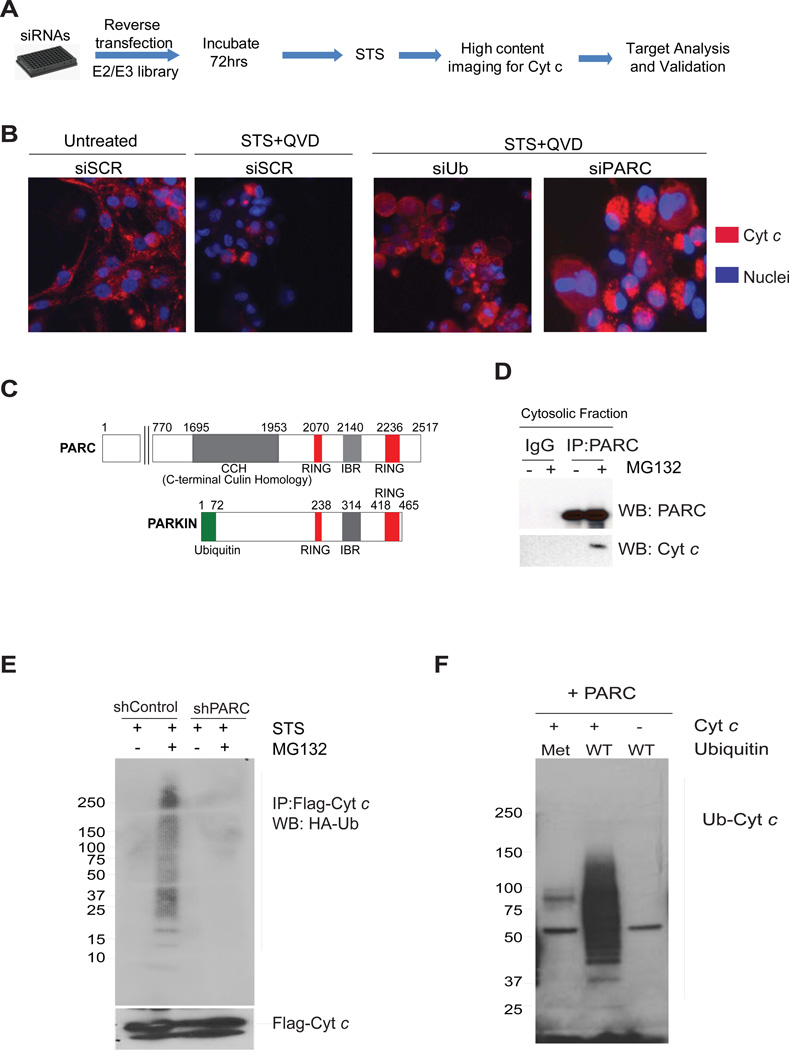
Figure 4.
(A) Flow diagram of the siRNA screen used for the identification of E3 ligases that target cyt c for degradation. (B) U87-MG cells were transfected with control scrambled (siSCR), ubiquitin (siUb)or PARC (siPARC) siRNAs and treated with staurosporine (1 µM) in the presence of QVD-fmk (25 µM) (see methods). The status of cyt c was assessed by immunostaining. (C) Schematic of PARC and Parkin proteins showing homologous regions. (D) Immunoprecipitation using cytosolic fractions demonstrates cyt c and PARC binding in U87-MG cells treated with staurosporine (STS) and MG132. (E) Ubiquitination assay using U87-MG control and shPARC knockdown cells transfected with Flag-cyt c and HA-ubiquitin, and then treated with staurosporine. Immunoprecipitates with Flag antibody (cyt c) were probed for HA-Ub by Western blot. (F) In vitro ubiquitination assay using immunoprecipitated PARC, recombinant cyt c and recombinant ubiquitin, either wild type (WT) or mutant. Recombinant mutant of ubiquitin (Met: Methylated ubiquitin-unable to form poly ubiquitin chains) was used as negative control. Data are representative of three experiments.