Abstract
BACKGROUND
Several lines of evidence suggest that proteasomes, the major nonlysosomal proteases in eukaryotes, are involved in the pathophysiology of various disease processes, including ischemia-reperfusion injury and trauma. Recently, we demonstrated that 26S proteasome activity is negatively regulated by adenosine triphosphate (ATP) and that proteasome activation during ischemia contributes to myocardial injury. The regulation of tissue proteasome activity by ATP and the potential of proteasomes as drug targets during hemorrhagic shock, however, are unknown. Thus, we evaluated the regulation of tissue proteasome peptidase activity and the effects of the proteasome inhibitor bortezomib in rat models of hemorrhagic shock.
METHODS
Series 1 includes animals (n = 20) hemorrhaged to a mean arterial blood pressure of 30mmHg for up to 45minutes. Series 2 includes animals hemorrhaged to a mean arterial blood pressure of 30 mm Hg for 30 minutes, followed by bortezomib (0.4 mg/kg) or vehicle administration (n =5 per group) and fluid resuscitation until 75 minutes. Series 3 includes animals that underwent 40% blood volume hemorrhage, followed by 2% blood volume hemorrhage every 15 minutes until death. Bortezomib (0.4 mg/kg) or vehicle were administered 15 minutes after the onset of hemorrhage (n = 6–7 per group). Vital signs were continuously monitored. The heart, lung, and pectoral muscle were analyzed for proteasome peptidase activities and levels of ATP, ubiquitin-protein conjugates, and cytokines (tumor necrosis factorα, interleukin 6, and interleukin 10).
RESULTS
In Series 1, proteasome peptidase activities in tissue extracts increased proportional to the decrease in tissue ATP concentrations during hemorrhagic shock. Activation of proteasome peptidase activity with decreases of the ATP assay concentration was also detectable in normal tissue extracts. In Series 2, systemic administration of bortezomib inhibited tissue proteasome activities but did not affect the physiologic response. In Series 3, bortezomib inhibited tissue proteasome activities, increased endogenous ubiquitin-protein conjugates, and prolonged survival time from treatment from 48.5 minutes in the control group to 85 minutes (p = 0.0012). Bortezomib treatment did not affect tissue cytokine levels.
CONCLUSION
Proteasome activation contributes to the pathophysiology of severe hemorrhagic shock. Pharmacologic inhibition of the proteasome may provide a survival advantage during lethal hemorrhagic shock.
Keywords: Bortezomib, resuscitation, ATP, 26S proteasome, male Lewis rats
The ubiquitin proteasome pathway of protein degradation (UPP) is the major nonlysosomal proteolytic system in eukaryotes. In this pathway, ubiquitin-protein ligase systems catalyze the covalent ligation of ubiquitin to proteins.1 Ubiquitylation serves then as the recognition signal for degradation of the target protein by the adenosine triphosphate (ATP)–dependent 26S proteasome. The 26S proteasome is formed from the 20S proteasome core particle, a cylinder-shaped multimeric protein complex, and either singly or doubly capped at its ends by a 19S regulator complex.1–4 While ATP is required for the assembly and function of the 26S proteasome complex, the 20S core particle alone is involved in the degradation of misfolded and damaged proteins, independent of ATP or ubiquitylation.5,6
It is well established that the UPP regulates essential cellular functions, and numerous lines of evidence suggest that it is also involved in the pathophysiology of various disease processes, including sepsis, burns, or trauma.7–15 Multiple proteasome inhibitors have been developed and are known to possess anti-inflammatory properties through inhibition of nuclear factor kappa-light-chain-enhancer of activated b cells (NFκB) activation.4,16–18 Proteasome inhibitors have been reported to diminish ischemia-reperfusion injury in various organs and to confer neuroprotection after intracerebral hemorrhage and traumatic brain injury.19–23 While most studies attributed beneficial effects of proteasome inhibitors to their anti-inflammatory actions, our recent findings suggested that proteasome inhibitors have additional direct tissue protective properties.24 We provided evidence that ATP negatively regulates 26S proteasome function and that activation of the myocardial 26S proteasome at low tissue ATP levels during cold ischemia contributes to organ damage, which can be attenuated by pharmacologic inhibition of the proteasome.24,25
It is well established that traumatic and hemorrhagic shock are associated with rapid changes in cellular ATP levels.26,27 In connection with our observation that the 26S proteasome may function as a cell destructive protease that is activated when the cellular energy supply declines,24 these data led to the hypothesis that 26S proteasome activation is a pathophysiologic mechanism, which also contributes to tissue injury after severe traumatic and hemorrhagic shock. Furthermore, the effects of proteasome inhibition during severe hemorrhagic shock and the early resuscitation period are unknown. Therefore, we performed a series of pilot studies in rat models of hemorrhagic shock to assess whether proteasome activation occurs with changes in tissue ATP concentrations and to provide initial information on the effects of bortezomib, a Food and Drug Administration approved proteasome inhibitor,28 during sublethal and lethal hemorrhagic shock.
MATERIALS AND METHODS
Animal Protocols
All procedures were performed according to National Institutes of Health Guidelines for Use of Laboratory Animals and approved by the Loyola Institutional Animal Care and Use Committee (IACUC). Male Lewis rats (bodyweight, 250–400 g; Harlan, Indianapolis, IN) were anesthetized with 100mg/kg ketamine, 10mg/kg xylazine intraperitoneal. This dose allowed the animals to be deeply sedated but able to breathe spontaneously throughout the experiment. After a midline laparotomy, the aorta was approached by a right medial visceral rotation. The aorta was instrumented with a 22-gauge angiocatheter for monitoring of blood pressure and blood withdrawal. The abdomen was then closed with monofilament suture. Core body temperature was maintained using warming lamps. After achieving stable baseline conditions (at least 5–10minutes after instrumentation), animals were randomized to one of the following conditions.
Series 1
Animals (n = 20) were hemorrhaged to a mean arterial blood pressure (MAP) of 30 mm Hg for up to 45 minutes. At 0, 15, 30, and 45 minutes, 5 animals were injected with cold (4°C) University of Wisconsin solution via the implanted catheter to induce cardioplegic arrest. Cardioplegic arrest was performed in this series to be able to assess changes in myocardial ATP levels.24 The hearts, lungs, and pectoral muscles were harvested and snap frozen in liquid nitrogen until further analyses.
Series 2
Animals (n = 10) were hemorrhaged to a MAP of 30 mm Hg for 30 minutes. At 30 minutes, 0.4mg/kg bortezomib (Velcade, Selleck Chemicals, Houston, TX; n = 5) or vehicle (isotonic sodium chloride solution, n = 5) was administered intraarterial (i.a.) in a total volume of 0.5 mL. This dose was selected based on the dosing of bortezomib in previous rodent studies.23,29 The animals were then resuscitated with isotonic sodium chloride solution until MAP returned to 70 mm Hg. MAP was then maintained at 70 mm Hg by continuous fluid administration for a total of 45 minutes, as required. At the end of the resuscitation period, the animals were euthanized by rapid exsanguination. The hearts, lungs, and pectoral muscles were harvested and snap frozen in liquid nitrogen until further analysis.
Series 3
Animals (n = 13) underwent withdrawal of 40% of the blood volume within 10 minutes. Blood was withdrawn at a rate of approximately 1 mL/min. At 15 minutes, 0.4mg/kg bortezomib (n = 7) or vehicle (isotonic sodium chloride solution, n = 6)was administered i.a. in a total volume of 0.5mL. The time point of drug/vehicle administration in this model was chosen to ensure that none of the animals dies before treatment, thus minimizing the use of animals. Thereafter, 2% of the blood volume was withdrawn every 15 minutes until death, as defined by asystole or disappearance of a pulse pressure. At the time of death, the hearts, lungs, and pectoral muscles were harvested and snap frozen in liquid nitrogen until further analyses.
In Series 1 to 3, arterial blood was collected in lithium heparin tubes (APP Pharmaceuticals, Schaumburg, IL) and used for blood gas analyses and analyses of routine laboratory parameters.
Blood Gas Analyses and Routine Laboratory Parameters
Measurements of pH, PCO2, PO2, hemoglobin, sodium, potassium, chloride, glucose, lactate, and bicarbonate were performed using a blood gas analyzer (Stat Profile pHOx Plus L, Nova Biomedical, Waltham, MA).
Tissue Extract Preparation
Snap frozen tissues were homogenized in 1/10 phosphate-buffered saline, pH 7.4 (1:5 wt/vol), centrifuged (16,600 g, 4°C, 30 minutes) and supernatants (extracts) aliquoted, as described.13,24 Protein concentrations in the tissue extracts were determined using the DC protein assay (Bio-Rad, Hercules, CA). All measurements in tissue extracts were standardized to total protein content.
ATP Assay
Tissues were homogenized in 1% TCA, centrifuged (2,000 g, 10 minutes, 4°C), and supernatants were collected. Supernatants were assayed for ATP using a bioluminescence assay (Invitrogen, Grand Island, NY), as described.24 For the calculation of the actual tissue ATP concentrations, the ATP concentration in extracts prepared from tissues before hemorrhage (t = 0 minute) were considered to equal a normal cellular ATP concentration.
Proteasome Peptidase Activity
Proteasome peptidase activities (total peptidase activity minus activity in the presence of the specific proteasome inhibitor epoxomicin) in tissue extracts were measured using the chymotryptic-like (CT-L) fluorogenic peptide substrate N-Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Enzo Life Sciences, Farmingdale, NY), as described.13,24,25 Incubation mixtures contained 50 µg of extract protein, 200 µM of the peptide substrate, and ATP at the actual tissue concentration. All enzyme assays were performed immediately after preparation of the tissue extracts to prevent from proteasome inactivation by freeze-thawing. Enzyme time progression curves showed linearity for 40 minutes.
Western Blots
Western blotting with anti-ubiquitin (Sigma, St. Louis, MO; and LifeSensors, Malvern, PA) and densitometric quantification of the chemiluminescence signals were performed as described.13,24,25 Anti-glyceraldehyde 3-phosphate dehydrogenase (Anti-GAPDH, Applied Biosciences, Foster City, CA) in combination with horseradish peroxidase-labeled anti-mouse (GE Healthcare, Burr Ridge, IL) were used as protein loading control. Chemiluminescence signals were detected with a Chemidoc imaging system and analyzed using the Quantity One gel analyses software (BioRad, Hercules, CA).
Enzyme-Linked Immunosorbent Assays
Interleukin 6 (IL-6), IL-10 and tumor necrosis factor α (TNF-α) were measured in tissue extracts with commercially available enzyme-linked immunosorbent assay kits (all from R&D Systems, Minneapolis, MN), according to the manufacturer’s protocols. Measurements were performed with specimens that had not been thawed previously after all animal experiments were completed.
Statistical Analysis
Data are presented as mean (SD) or median with minimum and maximum, as appropriate. Normal distribution was assessed with the Kolmogorov-Smirnov test. Normally distributed data were analyzed with Student’s t test or two-way repeated measures (mixed model) analysis of variance and Bonferroni’s post hoc test to correct for multiple testing. Data that did not pass the normality test (α= 0.05) were analyzed with the Mann-Whitney U-test or Kruskal-Wallace H test with Dunn’s multiple comparison posttest, as appropriate. Survival was plotted using the Kaplan-Meier method, and survival between the groups was compared with the log rank test. Statistical analyses, Spearman’s correlation analyses, and nonlinear regression analyses (one-phase exponential decay) were calculated with the GraphPad Prism program (GraphPad Software, La Jolla, CA). A two-tailed p < 0.05 was considered significant.
RESULTS
Proteasome Peptidase Activity During Hemorrhagic Shock (Series 1)
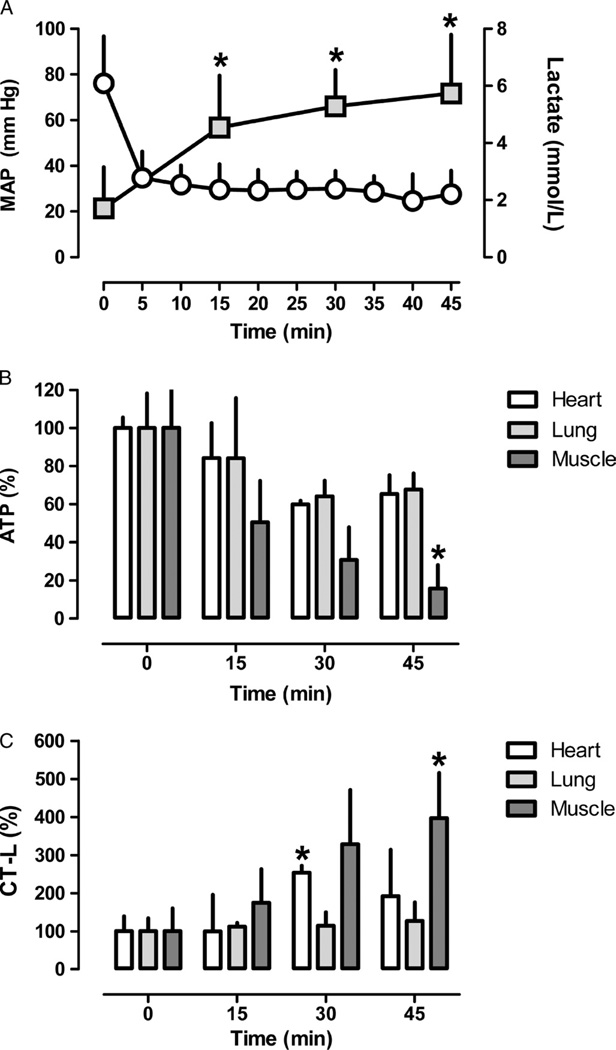
To assess alterations of proteasome peptidase activities in context of the tissue ATP content during hemorrhagic shock, we selected the heart and lung as representative central organs and skeletal muscle as representative peripheral organ. During hemorrhagic shock, arterial lactate levels increased from 1.7 (1.4) mmol/L at baseline to 5.7 (2.0) mmol/L at the end of the shock period (Fig. 1A). ATP levels decreased to 65% (17%) of control (t = 0 minute) at 45 minutes in the heart extracts, and to 68% (17%) in the lung extracts, respectively (Fig. 1B). In skeletal muscle extracts, ATP levels decreased to 16% (25%) of control at 45 minutes (Fig. 1B). For the measurements of proteasome peptidase activities in the tissue extracts, an assay concentration of 2 mM of ATP was used to reflect a normal cellular ATP concentration in lung and skeletal muscle30,31 and of 5 mM of ATP to reflect the normal ATP concentration in the heart.32–34 The assay concentration of ATP was then adjusted based on the percentage of decrease of the ATP concentration from baseline for proteasome peptidase activity measurements in extracts from tissues that were harvested during hemorrhagic shock. Under these conditions, proteasome peptidase activities increased to 253% (18%) of control at 30 minutes in heart extracts, to 127% (48%) of control at 45 minutes in lung extracts and to 397% (119%) of control at 45 minutes in skeletal muscle extracts (Fig. 1C).
Figure 1.
A, MAP (mm Hg, mean [SD]; open circles) and arterial lactate concentrations (mmol/L, mean [SD]; grey squares), n = 20, 15, 10, and 5 at 0, 15, 30, and 45 minutes, respectively. B, ATP concentrations in heart (open bars), lung (light grey bars), and pectoral muscle (dark grey bars) extracts. Data are given as percentage of control (t = 0 minute, mean [SD]). N = 5 per time point. C, CT-L proteasome peptidase activities in heart (open bars), lung (light grey bars), and pectoral muscle (dark grey bars) extracts measured at the actual tissue ATP concentration. Data are given as percentage of the control (t = 0 minute, mean [SD]). N = 5 per time point. *p <0.05 versus control (t = 0 minute).
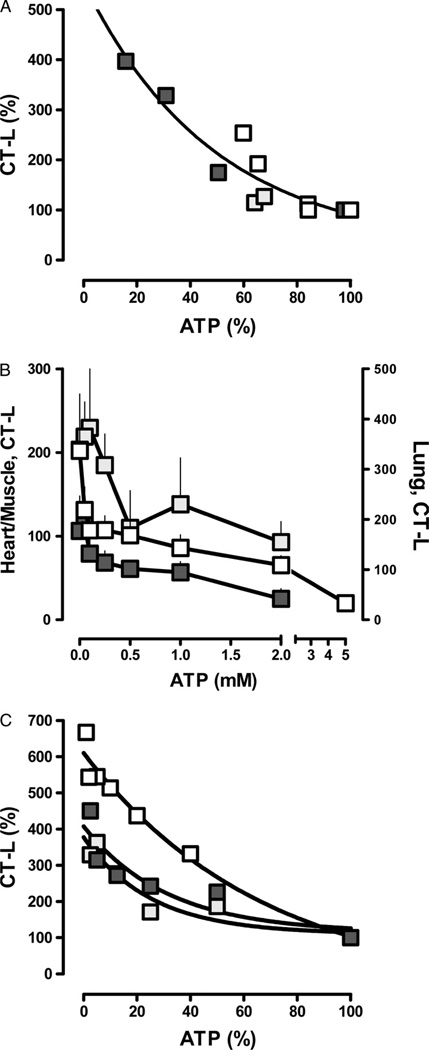
The proteasome peptidase activities correlated negatively with the determined ATP levels in the tissue extracts (Spearman’s r = −0.89). The tissue extract proteasome peptidase activities and ATP levels could be plotted according to a one-phase exponential decay when expressed as percentage of control (Fig. 2A; r2 = 0.89).
Figure 2.
A, Correlation between ATP levels and CT-L proteasome peptidase activities in heart (open squares), lung (light grey squares), and pectoral muscle (dark grey squares) extracts. Data from Figure 1B and C. r2 = 0.89. B, CT-L proteasome peptidase activities (pmol/min/50 µg) in normal heart (open squares), lung (dark grey squares), and pectoral muscle (dark grey squares) extracts measured at various ATP concentrations. Data are presented as mean (SD), n = 3 to 5. C, Correlation between assay ATP concentrations and CT-L proteasome peptidase activities. Data from B. Peptidase activities measured at 2mM (lung and pectoral muscle) and 5mM (heart) ATP were considered to equal 100%. r2 = 0.82 to 0.96.
When normal tissue extracts were assayed for proteasome peptidase activities at various ATP concentrations, reduction of ATP levels down to the low micromolar range resulted in a 2-fold to 10-fold increase of the proteasome peptidase activities with peak activities between 50µM and 100µM ATP (Fig. 2B). When proteasome peptidase activities were expressed as percentage of normal (2mM ATP for skeletal muscle and lung, 5mM ATP for heart), similar relationships between ATP levels and proteasome peptidase activities were observed as detected in tissue extracts from the organs harvested during hemorrhagic shock (Fig. 2C).
Proteasome Inhibition During Sublethal Hemorrhagic Shock and Resuscitation (Series 2)
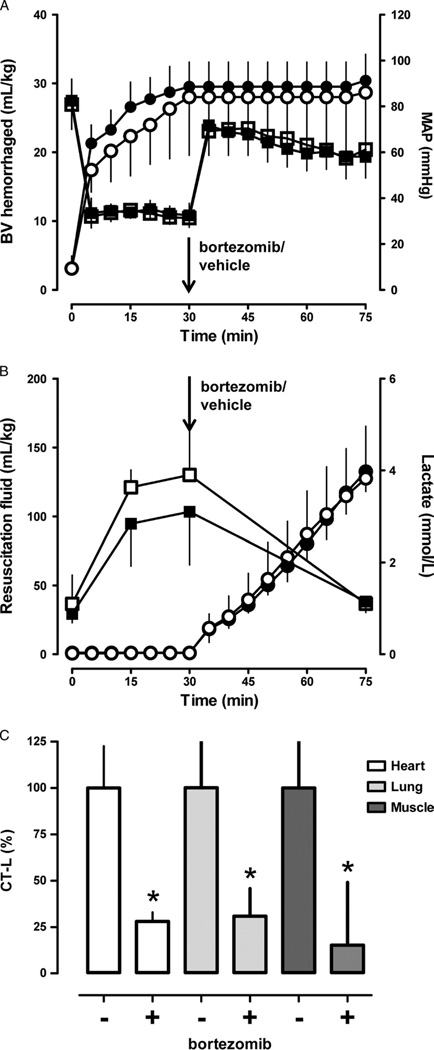
To determine the effects of proteasome inhibition during resuscitation from sublethal hemorrhage, bortezomib or vehicle were administered at the end of a 30minute shock period, before crystalloid fluid resuscitation. All animals survived the entire observation period. As shown in Figure 3A, the hemorrhage volume to achieve a MAP of 30 mm Hg for 30 minutes was comparable in both groups. All animals could be resuscitated to a MAP of 70 mm Hg (Fig. 3A) with comparable volumes of resuscitation fluids (Fig. 3B). In both groups, lactate levels increased to 3 mmol/L to 4 mmol/L at the end of the shock period and normalized with fluid resuscitation (Fig. 3B).
Figure 3.
Effects of bortezomib in a Wiggers model of sublethal hemorrhagic shock and resuscitation. Black symbols, bortezomib group (n = 5). Open symbols, vehicle group (n = 5). Data are presented as mean (SD). The arrows indicate the time point of bortezomib/vehicle administration. A, Blood volume (BV) hemorrhaged (mL/kg) to achieve a MAP of 30 mm Hg for 30 minutes (circles) and MAP (mm Hg; squares). B, Resuscitation fluid requirements (mL/kg) to maintain MAP of 70 mm Hg (circles) and arterial blood lactate levels (mmol/L; squares). C, CT-L proteasome peptidase activates in heart (open bars), lung (light grey bars), and pectoral muscle (dark grey bars) extracts in % of the vehicle group (100%). *p < 0.05 versus vehicle group.
There were no significant differences in any other of the measured physiologic parameters (not shown). Measurements of proteasome peptidase activities in extracts from tissues harvested at the end of the observation period documented 70% to 85% inhibition of the proteasome by bortezomib (Fig. 3C).
To further evaluate whether bortezomib treatment affected the inflammatory response in tissues, we then measured a selected panel of cytokines in tissue extracts from normal animals before hemorrhage (control) and in the tissue extracts at the end of the observation period after vehicle or bortezomib treatment. There were no differences in TNF-α, IL-6, and IL-10 skeletal muscle extract concentrations among the groups. As compared with control extracts, TNF-α and IL-6 concentrations in heart extracts were higher after hemorrhagic shock and resuscitation with bortezomib treatment, and IL-6 concentrations in lung extracts were higher after hemorrhagic shock and resuscitation with vehicle treatment, respectively. There were no statistically significant differences in tissue extract cytokine concentrations between animals after hemorrhagic shock and resuscitation when treated with vehicle or bortezomib (Table 1).
TABLE 1.
Cytokine Concentrations in Tissue Extracts
| Sublethal Hemorrhage | Lethal Hemorrhage | ||||
|---|---|---|---|---|---|
| pg/mg | Control | Vehicle | Bortezomib | Vehicle | Bortezomib |
| Heart | |||||
| TNF-α | 21 (4–60) | 56 (30–114) | 88 (36–175)* | 42 (35–57) | 43 (24–62) |
| IL-6 | 244 (49–626) | 570 (356–610) | 930 (251–1,588)* | 384 (323–557) | 353 (232–499) |
| IL-10 | 2.0 (1.7–8.2) | 2.2 (2.1–3.2) | 2.1 (1.8–2.6) | 2.3 (2.0–2.4) | 2.8 (1.8–3.2) |
| Lung | |||||
| TNF-α | 0.4 (0–3) | 2.9 (0–15) | 0.5 (0–7) | 1.6 (0–2.7) | 1.1 (0–2.2) |
| IL-6 | 19 (2–44) | 41 (25–64)* | 35 (21–85) | 17 (9–30) | 24 (16–51) |
| IL-10 | 1.4 (0–39) | 26 (13–42) | 19 (12–55) | 4 (3–8) | 5 (2–14) |
| Muscle | |||||
| TNF-α | 3.8 (0–8) | 0 (0–0.3) | 0.4 (0–1.5) | 5 (2–8) | 3 (2–12) |
| IL-6 | 99 (0–281) | 30 (25–76) | 65 (33–154) | 118 (60–204) | 171 (134–338) |
| IL-10 | 27 (4–114) | 20 (6–170) | 24 (6–59) | 47 (17–109) | 48 (34–83) |
p <0.05 versus control. Muscle, pectoral muscle. There were no statistically significant differences between the vehicle and bortezomib groups after sublethal and lethal hemorrhagic shock.
Data are presented as median (minimum–maximum). Control, n = 10 to 14; vehicle and bortezomib groups, n = 5 to 7 per group.
Proteasome Inhibition During Lethal Hemorrhagic Shock (Series 3)
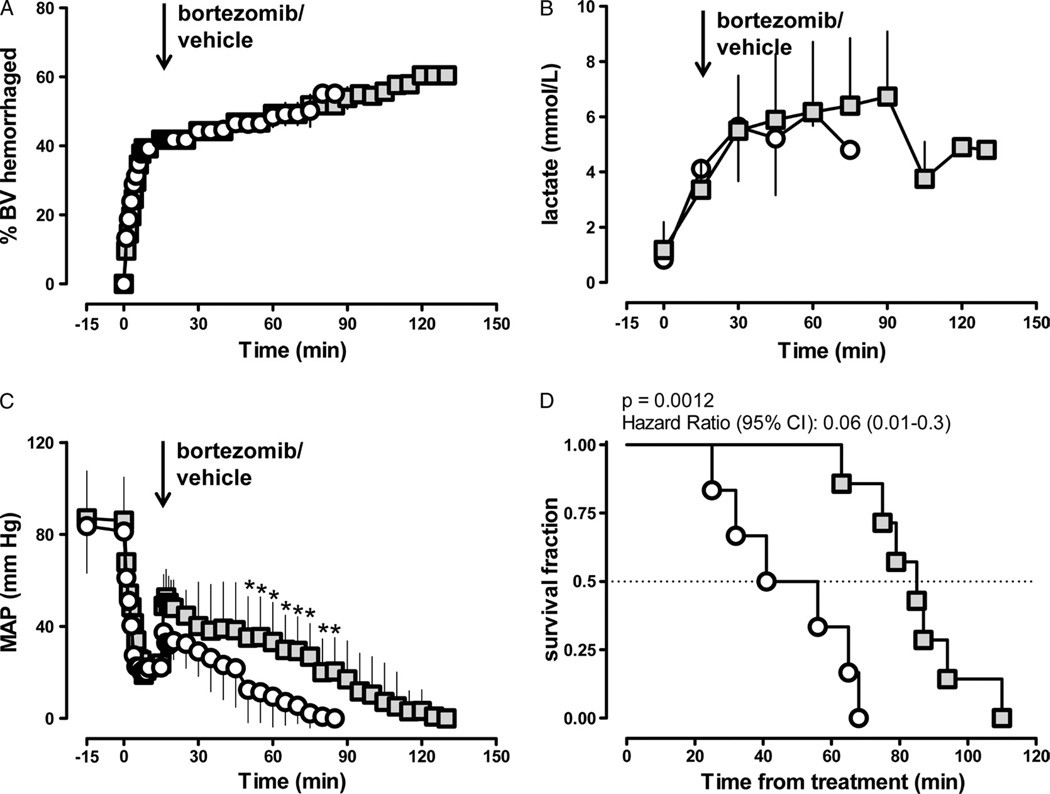
During lethal hemorrhagic shock, blood lactate levels increased to 6 mmol/L in vehicle and bortezomib-treated animals (Fig. 4A and B). MAP decreased to 20 mm Hg after withdrawal of 40% blood volume before drug administration in both groups (Fig. 4C). With bortezomib and vehicle administration, MAP recovered transiently to 40 mm Hg to 50 mm Hg and decreased thereafter. With bortezomib treatment, however, MAP could be maintained at significantly higher pressures (Fig. 4C), and median survival was significantly longer than with vehicle treatment (48.5 minutes with vehicle; 85 minutes with bortezomib; p = 0.0012; hazard ratio [95% confidence interval], 0.07 [0.013–0.34], Fig. 4D).
Figure 4.
Effects of bortezomib in a model of lethal hemorrhagic shock. Grey squares, bortezomib treatment (n = 7). Open circles, vehicle treatment (n = 6). Data are presented as mean (SD). The arrows indicate the time point of bortezomib/vehicle administration. A, Percentage of blood volume hemorrhaged. B, Arterial blood lactate levels (mmol/L). C, MAP (mm Hg). * p < 0.05 versus vehicle. D, Survival curve.
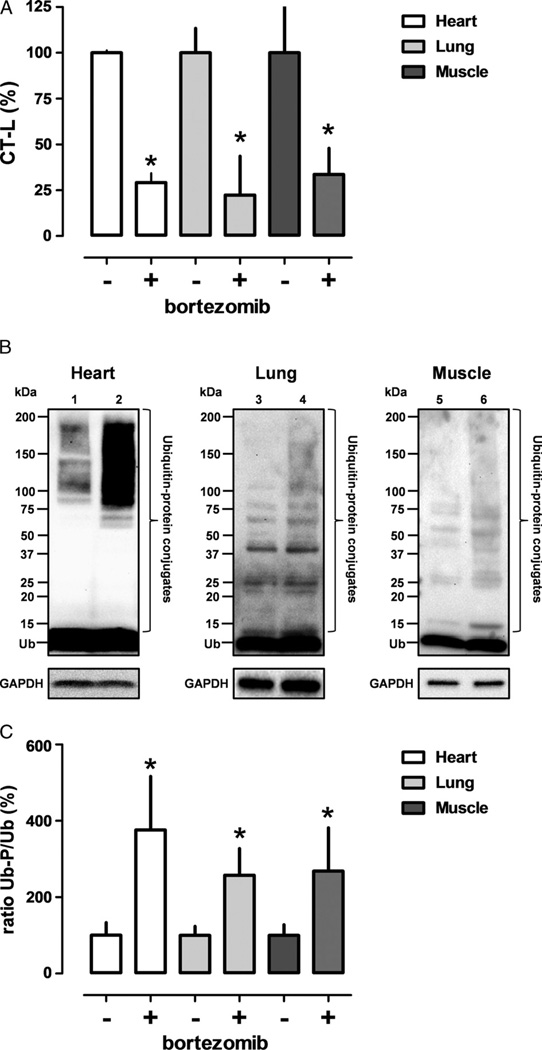
Similar to Series 2, proteasome peptidase activities in extracts from tissues harvested at the end of the experiment were significantly reduced with bortezomib treatment (Fig. 5A). To further confirm that this reduction in proteasome peptidase activity was functionally relevant, we then analyzed ubiquitin-protein conjugates in the tissue extracts by Western blotting with anti-ubiquitin.Figure 5B shows representative images of Western blotting experiments with tissue extracts, and Figure 5C shows the quantification of the chemiluminescence signals of the bands corresponding to free ubiquitin (8.6 kD) and ubiquitin-protein conjugates. After bortezomib treatment, endogenous levels of ubiquitin-protein conjugates were significantly increased in heart, lung, and skeletal muscle extracts, as compared with extracts from animals after vehicle treatment.
Figure 5.
Proteasome peptidase activities and ubiquitin-protein conjugates in tissue extracts after lethal hemorrhagic shock. Data are presented as mean (SD). A, CT-L proteasome peptidase activates in heart (open bars), lung (light grey bars), and pectoral muscle (dark grey bars) extracts in percentage of the vehicle group (100%). *p < 0.05 versus vehicle group. B, Ubiquitin immunoreactivity in tissue extracts (Lanes 1/2, heart; Lanes 3/4, lung; Lanes 5/6, pectoral muscle) after lethal hemorrhagic shock. Western blots with anti-ubiquitin (top) and anti-GAPDH (bottom, protein loading control). Lanes 1/3/5, vehicle treatment. Lanes 2/4/6, bortezomib treatment. Each lane contains 50 µg of protein. The migration position of protein standards is shown on the left. Ub, free ubiquitin. C, Quantification of the chemiluminescence signals for free ubiquitin and ubiquitin-protein conjugates from Western blotting experiments, as in B. The ratio between ubiquitin-protein conjugates (Ub-P) and free ubiquitin (Ub) is expressed as percentage of the vehicle-treated group. Open bars, heart; light grey bars, lung; dark grey bars, pectoral muscle. n = 3 to 5, mean (SD). *p < 0.05 versus vehicle group.
There were no significant differences in heart, lung and skeletal muscle extract cytokine concentrations among the groups (Table 1).
DISCUSSION
In this pilot study, we provide initial information on the regulation of the proteasome in context of the cellular ATP concentrations and the first assessment of the possible therapeutic properties of proteasome inhibitors during severe hemorrhagic shock in rat models. There are several new findings from the present study. First, organ proteasome activity is regulated by ATP and increases proportional to the decrease in tissue ATP levels during hemorrhage. Second, proteasome inhibition immediately before fluid resuscitation from sublethal hemorrhagic shock does not affect the physiologic response during the early resuscitation period. Third, proteasome inhibition during lethal hemorrhagic shock without fluid resuscitation improves hemodynamic stability and prolongs survival time.
The magnitude and time course of the changes in ATP concentrations in heart and lung extracts, which we detected in the present study, are in agreement with previous observations.27,35 The significant reduction of ATP levels in pectoral muscle, a red muscle,36 was unexpected because previous studies reported that ATP levels remained unchanged at comparable time points after hemorrhagic shock in resting white (gastrocnemius) and red (soleus) muscles.37,38 ATP levels in rat pectoral muscle during hemorrhage, however, have not been studied previously, and significant decreases in ATP levels have been described during early hemorrhagic shock in working muscle (diaphragm).38 As the pectoral muscle is an accessory muscle of respiration39 and rats were spontaneously breathing in our study, the rapid depletion of ATP levels likely reflects its metabolic activity. As such, our observations are consistent with the redistribution of blood flow away from peripheral toward central organs during hemorrhagic shock.
We demonstrated previously that chymotryptic-, tryptic-, and caspase-like 26S proteasome activities are activated as ATP levels decrease, whereas 20S proteasome activity is unaffected by variations of the ATP concentration.24 Subsequently, these findings have been confirmed independently and further supported by measurements in cell systems.40 Our findings on proteasome peptidase activities in tissue extracts during hemorrhagic shock and the observed regulation of proteasome peptidase activity in normal tissue extracts by ATP in the present study further support the concept that the cellular energy content determines the activation status of a major proteolytic system. In combination with previous reports on the regulation of proteasomes from various species and cellular sources,24,25,40 the present study suggests that this regulation of the 26S proteasome by ATP is a general pathophysiologic mechanism applicable to all organs and tissues.
As documented by enzyme activity measurements and Western blot analyses of endogenous ubiquitin-protein conjugates, the physiologic substrates of the 26S proteasome, the administered dose of bortezomib was able to inhibit the heart, lung, and skeletal muscle proteasome by 70% to 85%. This degree of proteasome inhibition is consistent with the pharmacokinetic properties and the pharamacodynamic effect of the standard clinical dose of bortezomib in patients (Food and Drug Administration Application number 021602-S015) and has been shown to produce beneficial effects after ischemia-reperfusion injury in animals.41,42 While we did not observe beneficial or adverse effects of bortezomib during the early resuscitation period after sublethal hemorrhagic shock, the same dose of drug administered during lethal hemorrhagic shock improved MAP and prolonged survival time. This may suggest that enhanced proteasomal degradation of cellular target proteins occurs as tissue ATP levels deplete progressively during exsanguination. This further implies that pathologic activation of the proteasome contributes to cardiovascular collapse during severe hemorrhagic shock.
As the UPP regulates a myriad of essential intracellular functions, including regulation of important signaling pathways and protein turnover, multiple mechanism likely contribute to the pathophysiologic effects of proteasome inhibition in the present study. Anti-inflammatory properties of bortezomib, however, seem unlikely to be responsible because its beneficial actions were detectable very early after hemorrhage and measurements of inflammation markers in tissues did not provide evidence for significant effects during this period. Thus, the identification of the molecular mechanisms leading to improved survival during lethal hemorrhagic shock remains a challenge and is beyond the scope of this pilot study.
Another limitation of the present study is the short observation period in the sublethal hemorrhagic shock model. Nevertheless, these experiments document that bortezomib treatment was not associated with obvious adverse effects on cardiovascular function, which have been reported after repetitive dosing.43
Taken together, the present study suggests that the tissue ATP concentration determines the activity of the 26S proteasome and that proteasome activation during lethal hemorrhagic shock contributes to cardiovascular collapse. In combination with previous studies in other model systems, the present study implies activation of the 26S proteasome as a new pathophysiologic mechanism, which contributes to organ system failure under conditions that are associated with decreases of the cellular ATP content. Proteasome inhibition during severe hemorrhagic shock may provide a survival benefit and, thus, could be useful for the exsanguinated trauma victim to buy time until fluid resuscitation and blood products become available. Based on the results of this pilot study and the organ protective effects of proteasome inhibitors in previous models of ischemia-reperfusion and traumatic brain injuries,19–21,23 further evaluation of the effects of proteasome inhibitors during longer periods of resuscitation from hemorrhagic shock and studies on the molecular mechanisms leading to improved outcomes seem justified.
Footnotes
This research has been presented, in part, at the 34th Annual Conference on Shock, Norfolk, Virginia, June 11–14, 2011, and was supported, in part, by NIHT32GM008750 and the Dr. Ralph and Marian Falk Medical Research Trust.
AUTHORSHIP
H.H.B., H.M.L., and Y.M.W. performed all experiments. H.H.B., R.L.G., and M.M. designed the experiments. H.H.B. and M.M. analyzed the data and wrote the article. All authors discussed the results and implications and commented on the article at all stages.
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry. 1990;29:10289–10297. doi: 10.1021/bi00497a001. [DOI] [PubMed] [Google Scholar]
- 3.Baumeister W, Walz J, Zuhl F, et al. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 4.Groll M, Huber R. Inhibitors of the eukaryotic 20S proteasome core particle: a structural approach. Biochim Biophys Acta. 2004;1695:33–44. doi: 10.1016/j.bbamcr.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Eytan E, Armon T, Heller H, et al. Ubiquitin C-terminal hydrolase activity associated with the 26 S protease complex. J Biol Chem. 1993;268:4668–4674. [PubMed] [Google Scholar]
- 6.Orlowski M, Wilk S. Ubiquitin-independent proteolytic functions of the proteasome. Arch Biochem Biophys. 2003;415:1–5. doi: 10.1016/s0003-9861(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 7.Tiao G, Hobler S, Wang JJ, et al. Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J Clin Invest. 1997;99:163–168. doi: 10.1172/JCI119143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobler SC, Williams A, Fischer D, et al. Activity and expression of the 20S proteasome are increased in skeletal muscle during sepsis. Am J Physiol. 1999;277:R434–R440. doi: 10.1152/ajpregu.1999.277.2.R434. [DOI] [PubMed] [Google Scholar]
- 9.Mansoor O, Beaufrere B, Boirie Y, et al. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin–dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci U S A. 1996;93:2714–2718. doi: 10.1073/pnas.93.7.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulteau AL, Lundberg KC, Humphries KM, et al. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 11.Keller JN, Huang FF, Zhu H, et al. Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2000;20:1467–1473. doi: 10.1097/00004647-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Manning EW, 3rd, Patel MB, Garcia-Covarrubias L, et al. Proteasome peptidase activities parallel histomorphological and functional consequences of ischemia-reperfusion injury in the lung. Exp Lung Res. 2009;35:284–295. doi: 10.1080/01902140802668823. [DOI] [PubMed] [Google Scholar]
- 13.Majetschak M, Patel MB, Sorell LT, et al. Cardiac proteasome dysfunction during cold ischemic storage and reperfusion in a murine heart transplantation model. Biochem Biophys Res Commun. 2008;365:882–888. doi: 10.1016/j.bbrc.2007.11.092. [DOI] [PubMed] [Google Scholar]
- 14.Seiffert M, Gosenca D, Ponelies N, et al. Regulation of the ubiquitin proteasome system in mechanically injured human skeletal muscle. Physiol Res. 2007;56:227–233. doi: 10.33549/physiolres.930966. [DOI] [PubMed] [Google Scholar]
- 15.Patel MB, Earle SA, Majetschak M. Dynamics of tissue ubiquitin pools and ubiquitin-proteasome pathway component activities during the systemic response to traumatic shock. Physiol Res. 2007;56:547–557. doi: 10.33549/physiolres.931068. [DOI] [PubMed] [Google Scholar]
- 16.Moore BS, Eustaquio AS, McGlinchey RP. Advances in and applications of proteasome inhibitors. Curr Opin Chem Biol. 2008;12:434–440. doi: 10.1016/j.cbpa.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng L, Mohan R, Kwok BH, et al. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 19.Kukan M. Emerging roles of proteasomes in ischemia-reperfusion injury of organs. J Physiol Pharmacol. 2004;55:3–15. [PubMed] [Google Scholar]
- 20.Yao JH, Li YH, Wang ZZ, et al. Proteasome inhibitor lactacystin ablates liver injury induced by intestinal ischaemia-reperfusion. Clin Exp Pharmacol Physiol. 2007;34:1102–1108. doi: 10.1111/j.1440-1681.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- 21.Tian XF, Zhang XS, Li YH, et al. Proteasome inhibition attenuates lung injury induced by intestinal ischemia reperfusion in rats. Life Sci. 2006;79:2069–2076. doi: 10.1016/j.lfs.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Sinn DI, Lee ST, Chu K, et al. Proteasomal inhibition in intracerebral hemorrhage: neuroprotective and anti-inflammatory effects of bortezomib. Neurosci Res. 2007;58:12–18. doi: 10.1016/j.neures.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Qu C, Mahmood A, Ning R, et al. The treatment of traumatic brain injury with velcade. J Neurotrauma. 2010;27:1625–1634. doi: 10.1089/neu.2010.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geng Q, Romero J, Saini V, et al. A subset of 26S proteasomes is activated at critically low ATP concentrations and contributes to myocardial injury during cold ischemia. Biochem Biophys Res Commun. 2009;390:1136–1141. doi: 10.1016/j.bbrc.2009.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker TA, Geng Q, Romero J, et al. Prolongation of myocardial viability by proteasome inhibition during hypothermic organ preservation. Biochem Biophys Res Commun. 2010;401:548–553. doi: 10.1016/j.bbrc.2010.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarrar D, Wang P, Chaudry IH. Metabolic disturbances in shock, and the role of ATP-MgCl2 and sex steroids. Med Princ Pract. 2004;13:2–9. doi: 10.1159/000074043. [DOI] [PubMed] [Google Scholar]
- 27.Kiang JG, Bowman PD, Lu X, et al. Geldanamycin prevents hemorrhage-induced ATP loss by overexpressing inducible HSP70 and activating pyruvate dehydrogenase. Am J Physiol Gastrointest Liver Physiol. 2006;291:G117–G127. doi: 10.1152/ajpgi.00397.2005. [DOI] [PubMed] [Google Scholar]
- 28.Twombly R. First proteasome inhibitor approved for multiple myeloma. J Natl Cancer Inst. 2003;95:845. doi: 10.1093/jnci/95.12.845. [DOI] [PubMed] [Google Scholar]
- 29.Hainz N, Thomas S, Neubert K, et al. The proteasome inhibitor bortezomib prevents lupus nephritis in the NZB/W F1 mouse model by preservation of glomerular and tubulointerstitial architecture. Nephron Exp Nephrol. 2012;120:e47–e58. doi: 10.1159/000334955. [DOI] [PubMed] [Google Scholar]
- 30.Gribble FM, Loussouarn G, Tucker SJ, et al. A novel method for measurement of submembrane ATP concentration. J Biol Chem. 2000;275:30046–30049. doi: 10.1074/jbc.M001010200. [DOI] [PubMed] [Google Scholar]
- 31.Ando T, Imamura H, Suzuki R, et al. Visualization and measurement of ATP levels in living cells replicating hepatitis C virus genome RNA. PLoS Pathog. 2012;8:e1002561. doi: 10.1371/journal.ppat.1002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beer M, Seyfarth T, Sandstede J, et al. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol. 2002;40:1267–1274. doi: 10.1016/s0735-1097(02)02160-5. [DOI] [PubMed] [Google Scholar]
- 33.Bottomley PA, Atalar E, Weiss RG. Human cardiac high-energy phosphate metabolite concentrations by 1D-resolved NMR spectroscopy. Magn Reson Med. 1996;35:664–670. doi: 10.1002/mrm.1910350507. [DOI] [PubMed] [Google Scholar]
- 34.Kostler H, Landschutz W, Koeppe S, et al. Age and gender dependence of human cardiac phosphorus metabolites determined by SLOOP 31P MR spectroscopy. Magn Reson Med. 2006;56:907–911. doi: 10.1002/mrm.21027. [DOI] [PubMed] [Google Scholar]
- 35.Kiang JG, Lu X, Tabaku LS, et al. Resuscitation with lactated Ringer solution limits the expression of molecular events associated with lung injury after hemorrhage. J Appl Physiol. 2005;98:550–556. doi: 10.1152/japplphysiol.00858.2004. [DOI] [PubMed] [Google Scholar]
- 36.Blanchaer MC, Van Wijhe M. Isozymes of lactic dehydrogenase in skeletal muscle. Am J Physiol. 1962;202:827–829. doi: 10.1152/ajplegacy.1962.202.5.827. [DOI] [PubMed] [Google Scholar]
- 37.Jennische E, Amundson B, Haljamae H. Metabolic responses in feline “red” and “white” skeletal muscle to shock and ischemia. Acta Physiol Scand. 1979;106:39–45. doi: 10.1111/j.1748-1716.1979.tb06367.x. [DOI] [PubMed] [Google Scholar]
- 38.Chaudry IH, Sayeed MM, Baue AE. Alterations in high-energy phosphates in hemorrhagic shock as related to tissue and organ function. Surgery. 1976;79:666–668. [PubMed] [Google Scholar]
- 39.Costa D, Cancelliero KM, Polacow ML, et al. Metabolic profile of respiratory muscles of rats with bleomycin-induced pulmonary fibrosis: relationship with oxidative stress. J Chin Clin Med. 2008;3:123–132. [Google Scholar]
- 40.Huang H, Zhang X, Li S, et al. Physiological levels of ATP negatively regulate proteasome function. Cell Res. 2010 Dec;20(12):1372–1385. doi: 10.1038/cr.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu X, Huang S, Patterson E, et al. Proteasome degradation of GRK2 during ischemia and ventricular tachyarrhythmias in a canine model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289:H1960–H1967. doi: 10.1152/ajpheart.00328.2005. [DOI] [PubMed] [Google Scholar]
- 42.Huang S, Patterson E, Yu X, et al. Proteasome inhibition 1 h following ischemia protects GRK2 and prevents malignant ventricular tachyarrhythmias and SCD in a model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2008;294:H1298–H1303. doi: 10.1152/ajpheart.00765.2007. [DOI] [PubMed] [Google Scholar]
- 43.Nowis D, Maczewski M, Mackiewicz U, et al. Cardiotoxicity of the anticancer therapeutic agent bortezomib. Am J Pathol. 2010;176:2658–2668. doi: 10.2353/ajpath.2010.090690. [DOI] [PMC free article] [PubMed] [Google Scholar]