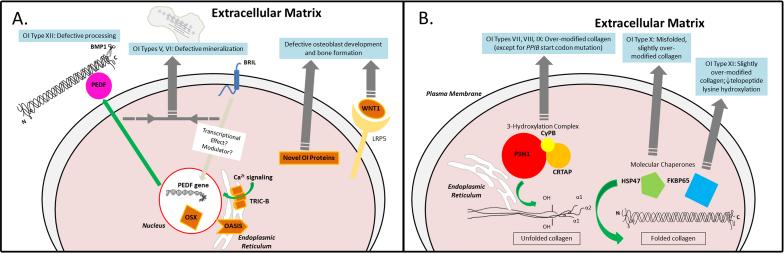
Figure 1.
Abnormal molecular mechanisms in OI types caused by defects in collagen-related molecules. Dark grey arrows and the text in blue boxes show the aberrant mechanism in most OI cases linked to defects in the specific protein. A) Green arrow shows the normal secretion of PEDF (and subsequent collagen binding) after transcription in response to effects of BRIL protein and a possible modulator. The novel OI proteins all affect osteoblast development: specifically, nuclear OSTERIX affects osteoblast differentiation genes, the trimeric TRIC-B channel affects intracellular Ca2+ signaling, the ER-stress transducer OASIS modulates Col1 transcription when nuclear, and WNT1 binds LRP5 to affect bone formation. B) Green arrows show the normal effect of the proteins on collagen. The ER resident complex of CRTAP/P3H1/CyPB 3-hydroxylates specific prolines on the α1(I) and α2(I) chains, while chaperones contribute to normal folding of the collagen trimer.