Figure 3. PKM2 K433 Acetylation Is Associated with Cell Growth Stimulation.
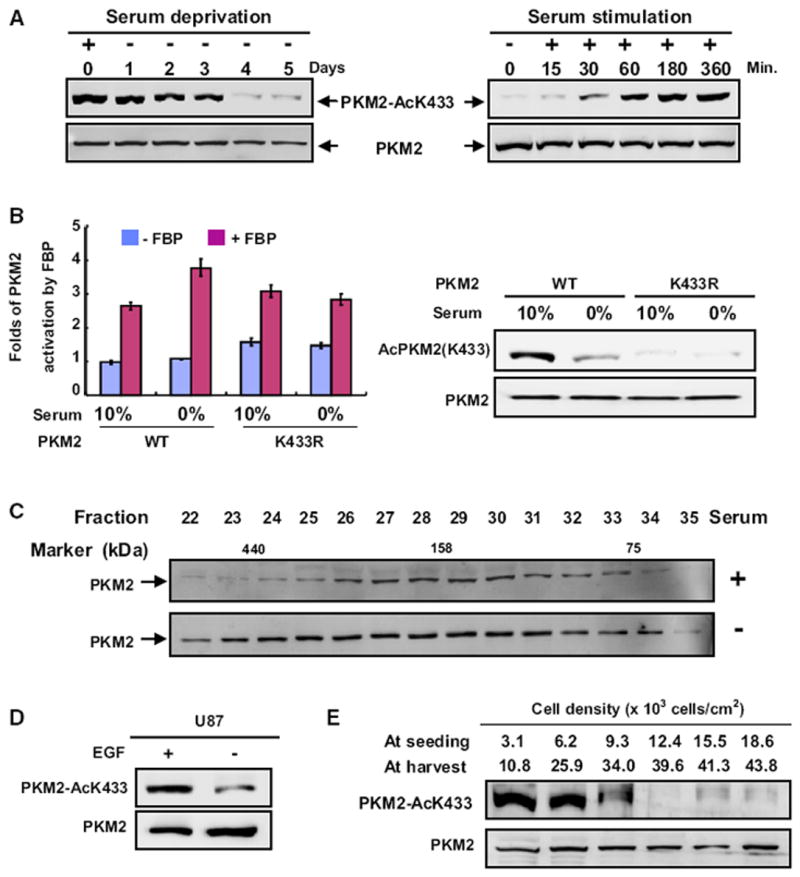
(A) Serum deprivation and stimulation decrease and increase PKM2 K433 acetylation, respectively. H1299 cells were serum starved (0% FBS) for different times or stimulated with the addition of 10% FBS after 2 days of serum starvation. Total cell lysates were analyzed by western analysis as indicated.
(B) Serum starvation enhances FBP activation of the pyruvate kinase activity of wild-type, but not the K433R mutant PKM2. WT and K433R mutant PKM2 proteins were immunopurified from transfected 293T cells cultured in the presence of 0% or 10% FBS, followed by enzyme activity in the presence or absence of FBP. The mean values of triplicates ± SD are presented.
(C) Serum deprivation prevents PKM2 dimerization. H1299 cells were cultured with or without serum for two days, and cell lysates were separated by gel filtration, followed by western analysis.
(D) EGF promotes K433 acetylation of PKM2. U87 cells were starved for 24 hr with 0.5% serum, followed by treatment with EGF (100 ng/ml) for 6 hr, and the PKM2 protein and K433 acetylation levels were determined by western analysis.
(E) PKM2 K433 acetylation inversely correlates with cell density. H1299 cells were seeded at different densities as indicated, cultured for 2.5 days, and the levels of both PKM2 and K433-acetylated PKM2 were analyzed by western blotting. See also Figure S2.
