Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) promotes neurite outgrowth, reduces proliferation and inhibits apoptosis of PC12 cells. We have partially characterized the transcriptome changes induced by PACAP after 6 h of treatment, when commitment to differentiation has occurred. Here, we have investigated the effects of a 6-h treatment with PACAP (10−7 m) in the presence of cycloheximide (5 μm) to identify, via superinduction, components of the transitional transcriptome initially induced by PACAP and potentially participating in the regulation of late-response genes required for differentiation. Approximately 100 new transcripts were identified in this screen, i.e. as many individual genes as make up the 6-h PACAP differentiation transcriptome itself. Six known transcripts in this cohort were then measured at several time points between 0 and 6 h by real-time PCR to determine whether these transcripts are induced early following PACAP treatment in the absence of cycloheximide, and therefore may be of functional importance in differentiation. Five out of the six transcripts were indeed induced by PACAP alone soon (between 30 min and 3 h) after cell treatment. β-Cell translocation gene 2, antiproliferative (Btg2), serum/glucocorticoid-regulated kinase (Sgk), nuclear factor for the κ chain of B-cells (NFκB), seven in absentia homologue 2 (Siah2) and FBJ osteosarcoma related oncogene (Fos) showed a 2.5–200-fold induction by PACAP between 15 min and 3 h, and mRNA levels returned either to baseline or near baseline after 6 h. This work provides new information concerning genes whose transient regulation early after PACAP exposure may contribute to the expression of the differentiated transcriptome in PC12 cells, and should help to elucidate the molecular mechanisms involved in the control of nerve cell survival and differentiation.
Keywords: cycloheximide, early response genes, microarray, PACAP, transitional transcriptome
The PC12 cell line was selected from a rat adrenal pheochromocytoma (PC) based on its ability to differentiate (cease proliferation and extend branching varicose processes) in the presence of nerve growth factor (NGF; Greene and Tischler 1976). Treatment of PC12 cells with pituitary adenylate cyclase-activating polypeptide (PACAP) also induces growth arrest, promotes neuritogenesis (Deutsch and Sun 1992), changes cell size (Ravni et al., in preparation), inhibits apoptosis (Vaudry et al. 2000) and increases the expression of a battery of genes specific to neuroendocrine cell secretory function. We characterized the transcriptome of PC12 cells committed to PACAP-induced differentiation by collecting RNA samples after a 6-h exposure to PACAP, sufficient for the unfolding of the differentiation program over a subsequent 48–72-h period (Vaudry et al. 2002a). RNA from PACAP-treated and -untreated cells was hybridized using two-colour technology on 15 000-element cDNA microarray slides (Vaudry et al. 2002a). These experiments revealed 73 genes robustly regulated by PACAP, with 71% of the transcripts increased and 29% decreased in abundance.
We then measured the dependence on new protein synthesis of both up- and down-regulated messenger RNA by re-examining PACAP induction in the presence of cycloheximide (Wettstein et al. 1964), to determine whether de novo protein synthesis was required for the transcription of genes regulated by PACAP. Most of the transcripts induced within a 6-h period did not themselves require the induction of new protein synthesis. However, some did, including transcripts such as annexin A2 and actin-related protein 2/3 complex, which are likely to be important for the eventual production of the differentiated PC12 cell phenotype (Vaudry et al. 2002a). These results suggested that identification of transitional transcripts – those encoding gene products required for the unfolding of the differentiation program but not part of the final differentiated cell transcriptome – might be required for a full understanding of the molecular events of PC12 cell differentiation by PACAP.
In addition to inhibiting protein synthesis required to effect neurite elongation and other cellular changes attending differentiation, cycloheximide and similar agents normally stabilize short-lived mRNA, such as those encoding immediate early genes like activity-regulated cytoskeleton-associated protein (Arc) and FBJ osteosarcoma-related oncogene (Fos), so that these normally transiently expressed transcripts can be detected for longer periods following their initial induction (Vaudry et al. 1998; Ichikawa et al. 2003). These transcripts also often exhibit mRNA superinduction in the presence of a translational inhibitor (Vaudry et al. 1998). These messengers are usually associated with rapid mRNA turnover and often contain AU-rich elements (Caput et al. 1986), which are thought to confer instability, in their 3′ untranslated regions (3′UTR; Roshak et al. 1996).
Superinduction is commonly attributed to decreased mRNA turnover, although increased nuclear signalling may also explain this effect (Edwards and Mahadevan 1992). Based on this concept of superinduction, microarray analyses were conducted to identify genes that are regulated after a 6-h treatment with PACAP in the presence of cycloheximide, but not initially shown to be up-regulated at the 6-h time point by PACAP alone. These transcripts represent potential immediate early genes, the protein products of which may act in a transient manner within the first 6 h of treatment to further transactivate genes effector for neurite extension, morphological alterations, electrical excitability, cessation of proliferation, protection from apoptosis or newly synthesized prohormones, the secretory vesicle payloads of differentiated neuroendocrine cells.
Following the detection of transcripts elevated after 6 h of treatment with PACAP in the presence of cycloheximide, a potential involvement of members of this cohort in the PACAP transitional transcriptome was tested by examining the regulation of these transcripts by PACAP alone between 0 and 6 h of treatment. Our results indicate that the cycloheximide microarray screen, paired with a detailed time-resolved analysis of individuals transcripts after exposure to neurotrophic factors is a comprehensive approach to identify transcripts of the transitional transcriptome of differentiating neuroendocrine cells.
Materials and methods
Cell culture and treatments
PC12-G cells were plated at a density of 70 000 cells/mL (140 cells/mm2) on poly-l-lysine-coated plates and cultured at 37°C in 10% CO2 and 90% air atmosphere. Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 7% heat-inactivated fetal bovine serum, 7% horse serum, 2.5% HEPES, 1 × glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin antibiotic. When necessary, cells were pre-incubated in either the absence or the presence of cycloheximide (5 μm) 30 min before treatment with either control medium or PACAP (10−7 m).
RNA extraction, probe preparation and microarray hybridization
After 6 h of treatment, RNA samples were harvested from the cells with Trizol reagent and further purified using the RNeasy Maxi Kit (Qiagen, Valencia, CA, USA). Fluorescently labelled cDNAs were synthesized from RNA by oligo(dT)-primed reverse transcription in the presence of either Cy3- or Cy5-2′deoxy-uridine 5′triphosphate (dUTP). After denaturation, purified Cy3/Cy5-labelled probes were combined and hybridized on a microarray slide containing over 15 000 mouse cDNA sequences (Tanaka et al. 2000) in the presence of 2 × Denhart’s solution, 3.3 × saline sodium citrate (SSC) and 0.5% sodium dodecyl sulfate (SDS) in a humidified chamber at 65 °C overnight. Prior to scanning (Agilent Technologies, Foster City, CA, USA), slides were successively washed at room temperature (22°C) in 0.5 × SSC/0.1% SDS for 2 min, 0.5 × SSC/0.01% SDS for 2 min and 0.06 × SSC for 2 min. Image analysis was performed with the IPLab software (Scanalytics, Fairfax, VA, USA). The two fluorescent images (red and green channels) obtained from the scanner constituted the raw data from which differential gene expression ratio and quality control values were calculated. All data were entered into a relational database, using the FileMakerPro 5 software (FileMaker, Santa Clara, CA, USA). Each spot on the microarray was assessed for quality control in the IPLab software. Any genes regulated in self–self hybridizations (either control vs. control or PACAP vs. PACAP hybridizations) were excluded from further consideration.
Reverse transcription and real-time PCR
Transcripts used for real-time PCR were extracted using the same protocol as for the microarray, except that during RNA purification DNA was removed by incubation of the samples with DNAse (Qiagen). cDNAs were synthesized from 4 μg of total RNA with ImProm II Promega kit (Promega, Charbonnières-les-Bains, France). Real-time PCR was performed on obtained cDNA in the presence of 1 × SYBR Green Mastermix (Applied Biosystems, Courtaboeuf, France) containing preset concentrations of dNTPs, MgCl2 and buffers, along with adequate concentrations of reverse and forward primers (Table 1).
Table 1.
Information about the primers used for conventional and real-time PCR
| Foward primer | Reverse primer | Amplicon size (bp) |
Tm (°C) | Final concentration (nm) |
|
|---|---|---|---|---|---|
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | ||||
| CAGCCTCGTCTCATAGACAAGATG | CAATGTCCACTTTGTCACAAGAGAA | 106 | 81 | 300 | |
| Fos | FBJ osteosarcoma related oncogene | ||||
| GCCAAGTGCCGGAATCG | AGTTGATCTGTCTCCGCTTGGA | 62 | 83 | 300 | |
| Btg2 | β-cell translocation gene 2, antiproliferative | ||||
| CGAGCAGAGACTCAAGGTTTTCA | ATAGCCGGAGCCCTTGGA | 103 | 81 | 300 | |
| Siah2 | Seven in absentia 2 | ||||
| AAGGTCGCCTCGGCAGTT | GGACGGTATTCACAGATGTCTTCA | 110 | 81 | 300 | |
| Sgk | Serum/glucocorticoid regulated kinase | ||||
| GGGACAACGTCCACCTTCTGT | CAGGCCATAGAGCATCTCATACAA | 129 | 84 | 300 | |
| NFκB |
Nuclear factor of kappa light chain gene
enhancer in B-cells, p105 |
||||
| AGGATTTCGATTCCGCTACGT | CCAACTGAACGATAACCTTTGCA | 140 | 81 | 300 | |
| Adnp | Activity-dependent neuroprotective protein | ||||
| TTGGGTTGGAATACTGTAAAGAACATATA | CCGATAGTCCTGATTTTTTGTAAGAGA | 134 | 74 | 500 | |
| PAC1 | PAC1 receptor | ||||
| CCCTGACTGCTCTCCTCCTGCTGCCTAT | CAGGGCAGCTCACAAGGACCATCTCACC | 213 | 82 | 300 | |
| PAC1 | PAC1 receptor * | ||||
| CTTGTACAGAAGCTGCAGTC | GGTGCTTGAAGTCCATAGTG | short 281 | 80 | 1000 | |
| hop 365 | 81 | ||||
| hip-hop 449 | 81 | ||||
| VPAC1 | VPAC1 receptor * | ||||
| GCCCCCATCCTCCTCTCCATC | TCCGCCTGCACCTCACCATTG | 298 | 81 | 1000 | |
| VPAC2 | VPAC2 receptor * | ||||
| GTCACCTTTGCCCTCTCCATCA | GCCTCTCCACCTTCTTTTCAGT | 296 | 80 | 1000 | |
Primers used for conventional PCR.
Conventional RT-PCR
PCR analysis of the PACAP receptors expressed in PC12-G cells was conducted using the same cDNA as for real-time PCR. RNAs isolated from hypothalamus and lung tissues were also added as positive controls for PACAP specific receptor (PAC1), PACAP and VIP receptor 1 (VPAC1) and PACAP and VIP receptor 2 (VPAC2) expression. Amplification of the PAC1, VPAC1 and VPAC2 receptors was conducted as previously described (Basille et al. 2000). The pairs of primers design to amplify the PAC1, VPAC1 and VPAC2 mRNAs are indicated in Table 1. The specificity and size of the amplicons was verified after migration on a 3% agarose gel in TBE and visualized under ultraviolet illumination after ethidium bromide staining.
Statistical analysis
For microarray experiments, data are presented as mean ± SEM from three independent experiments for control vs. PACAP plus cycloheximide, and from 12 independent experiments for control vs. PACAP. For real-time PCR, data are presented as mean ± SEM from three independent experiments performed in triplicate. Statistical analyses of the data were performed using one-way analysis of variance followed by a Tukey’s multicomparison post-test.
Results
Effects of PACAP plus cycloheximide on gene expression in PC12 cells
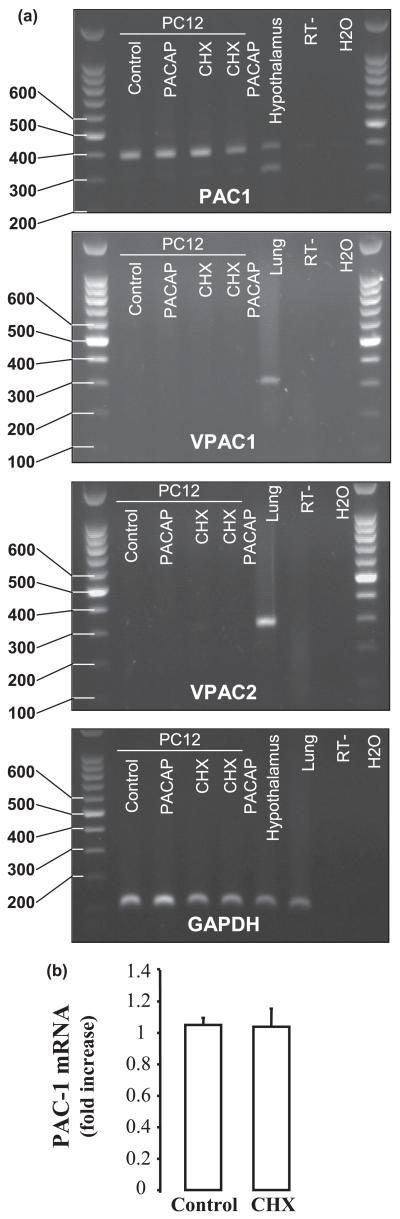
The extensive neurite sprouting seen 48 h after continuous exposure to PACAP can also be observed 48 h later, even if PC12 cells only receive an initial 6-h exposure to PACAP (Vaudry et al. 2002a). This suggests that some genes must be regulated very rapidly in these cells. In order to identify some early mRNA that would be induced transiently, PC12 cells were incubated with PACAP in the presence of cycloheximide. In control conditions, PC12-G cells express the hop and hip-hop variants of the PAC1 receptor but none of the VPAC receptors (Figs 1a-d). Treatment with cycloheximide does not affect the expression pattern of the PACAP receptors (Figs 1a-d) and does not change the expression level of the PAC1 receptor (Fig. 1e), which indicates that the results observed are not caused by a modification of the PACAP receptors. After 6 h of treatment, 166 transcripts were induced by at least two-fold. Among these genes, 106 had not been previously found to be regulated by PACAP alone at 6 h. Eight exhibited an average level of activation by PACAP plus cycloheximide that exceeded 10-fold (Table 2). According to the previous microarray experiments, some of the transcripts activated by PACAP plus cycloheximide such as serum/glucocorticoid-regulated kinase (Sgk) were not increased at all by PACAP alone at 6 h, whereas others, including either Cbp/p300-interacting transactivator with Glu/Asp-rich carboxterminal domain 1 (Cited1) or Fos, were increased by between 1.7- and 1.9-fold, which remains under the threshold limit of a two-fold induction that was applied to the previous analysis (Table 2). The highest induction by PACAP plus cycloheximide was observed for β-cell translocation gene 2, antiproliferative (Btg2; a 21-fold increase) followed by those observed for early growth response 1 (Egr1), Cited1 and Fos (Table 2). In contrast to the components of the transitional transcriptome discussed here, up-regulation of some classical PACAP-induced transcripts such as tyrosine hydroxylase mRNA, seen induced by PACAP alone in PC12-G cells, are not enhanced in the presence of cycloheximide suggesting that these mRNAs are probably regulated in an indirect manner.
Fig. 1.
Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) receptors mRNA in PC12 cells. (a) Expression of PAC1, VPAC1, VPAC2 and GAPDH receptor isoforms in either the absence or the presence of PACAP and/or cycloheximide. cDNA from hypothalamus and lung tissues were used as positive controls for PAC1 and VPAC receptors, respectively. (b) Quantification by real-time PCR of the effect of cycloheximide on PAC1 receptor expression after 30 min of treatment with cycloheximide.
Table 2.
Functional classification of the genes induced by a 6-h treatment with pituitary adenylate cyclase-activating polypeptide (PACAP) plus cycloheximide, but not by PACAP alone
| Gene name | Ug cluster | GB ID | Control vs. CHX + PACAP |
Control vs. PACAP |
|---|---|---|---|---|
| Development/physiological process/cellular process/regulation of biological process | ||||
|
| ||||
| β-cell translocation gene 2, antiproliferative (Btg2) | Mm.239605 | C87946 | 21.54 | 1.72 |
|
Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal
domain 1 (Cited1) |
Mm.2390 | AU019144 | 15.21 | 1.86 |
| FBJ osteosarcoma related oncogene (Fos) | Mm.246513 | AU015150 | 13.50 | 1.90 |
| Growth arrest and DNA-damage-inducible 45 gamma (Gadd45g) | Mm.281298 | C86281 | 7.86 | 1.14 |
| Jumonji, AT rich interactive domain 2 (Jarid2) | Mm.25059 | C80019 | 5.12 | 1.23 |
| Nuclear factor for kappa chain of B-cells | Mm.256765 | AU024604 | 4.50 | 1.15 |
| Signal transducer and activator of transcription 3 (Stat3) | Mm.249934 | AU043374 | 3.78 | 2.03 |
| DNA methyltransferase 3B (Dnmt3b) | Mm.89772 | AW537957 | 2.58 | 1.37 |
| Development/physiological process/behaviour/cellular process | ||||
|
| ||||
|
Platelet-activating factor acetylhydrolase, isoform 1b, beta1 subunit
(Pafah1b1) |
Mm.56337 | AU021752 | 3.11 | 1.57 |
| Development/physiological process/cellular process | ||||
|
| ||||
| Seven in absentia 2 (Siah2) | Mm.2847 | AU017586 | 6.35 | 1.21 |
| Tescalcin (Tesc) | Mm.273285 | AU023528 | 3.51 | 1.16 |
| Guanine nucleotide binding protein, alpha 13 (Gna13) | Mm.193925 | AW556943 | 2.74 | 1.17 |
| Nucleoporin 50 (Nup50) | Mm.28379 | AW556935 | 2.74 | 1.33 |
| Wingless-related MMTV integration site 4 (Wnt4) | Mm.20355 | AW549398 | 2.59 | 0.98 |
| Tousled-like kinase 2 (Arabidopsis) (Tlk2) | Mm.126976 | AW553714 | 2.50 | 1.08 |
| Development/physiological process | ||||
|
| ||||
| SID1 transmembrane family, member 2 (Sidt2) | Mm.200859 | AU046146 | 6.94 | 1.24 |
| Tuftelin interacting protein 11 (Tfip11) | Mm.172947 | AW547124 | 4.52 | 1.35 |
| Development/cellular process | ||||
|
| ||||
| Plakophilin 2 (Pkp2) | Mm.2252 | AW538171 | 12.11 | 1.12 |
| SKI-like (Skil) | Mm.15406 | AU014590 | 7.11 | 1.37 |
| Myeloid differentiation primary response gene 116 (Myd116) | Mm.4048 | AW536864 | 4.77 | 1.43 |
| Interferon-related developmental regulator 1 (Ifrd1) | Mm.168 | C87178 | 4.67 | 0.96 |
| Development | ||||
|
| ||||
| Ring finger protein 2 (Rnf2) | Mm.31512 | AW539513 | 2.27 | 1.15 |
| Physiological process/cellular process/regulation of biological process | ||||
|
| ||||
| Early growth response 1 (Egr1) | Mm.181959 | AU017579 | 16.58 | 1.92 |
| RIKEN cDNA 4930563E22 gene (Med31) | Mm.159496 | AU045064 | 5.08 | 0.94 |
| Activating transcription factor 3 (Atf3) | Mm.2706 | C86078 | 3.76 | 1.31 |
| YY1 transcription factor (Yy1) | Mm.3868 | AU017017 | 3.15 | 1.30 |
| CREB binding protein (Crebbp) | Mm.132238 | AW552828 | 2.80 | 1.16 |
| Acyl-CoA synthetase long-chain family member 4 (Acsl4) | Mm.143689 | AU019232 | 2.69 | 1.67 |
| GTPase activating RANGAP domain-like 1 (Garnl1) | Mm.292180 | AW548663 | 2.64 | 0.95 |
| Activity-dependent neuroprotective protein (Adnp) | Mm.201322 | AW554081 | 2.51 | 1.23 |
| General transcription factor IIF, polypeptide 1 (Gtf2f1) | Mm.24632 | AW556946 | 2.48 | 1.20 |
| Physiological process/cellular process | ||||
|
| ||||
| ADP-ribosylation factor-like 4 (Arl4) | Mm.12723 | AU021341 | 11.71 | 1.21 |
| Serum/glucocorticoid regulated kinase (Sgk) | Mm.28405 | AU042681 | 10.52 | 1.06 |
| RIKEN cDNA 2810012H18 gene (2810012H18Rik) | Mm.281741 | AU044772 | 4.49 | 1.26 |
| RIKEN cDNA 9130230 N09 gene (B3gnt1) | Mm.258094 | AU024115 | 4.33 | 1.30 |
| Paraspeckle protein 1 (Pspc1) | Mm.20129 | AU045828 | 4.25 | 1.03 |
|
Protein tyrosine phosphatase, receptor-type, F interacting protein, binding
protein 2 (Ppfibp2) |
Mm.2817 | AU041064 | 4.08 | 1.43 |
| Prostaglandin I2 (prostacyclin) synthase (Ptgis) | Mm.2339 | AW559113 | 3.92 | 1.18 |
| SAR1a gene homologue 1 (S. cerevisiae) (Sara1) | Mm.6698 | AW544555 | 3.68 | 1.37 |
| Protein tyrosine phosphatase 4a2 (Ptp4a2) | Mm.193688 | C88125 | 3.60 | 1.33 |
| Centromere autoantigen A (Cenpa) | Mm.290563 | AU021358 | 3.52 | 1.29 |
| RIKEN cDNA A130048E20 gene (Rev3l) | Mm.288788 | AW547620 | 3.36 | 0.95 |
| ELAV (embryonic lethal, abnormal vision, Drosophila)-like 1 (Hu antigen R) (Elavl1) | Mm.119162 | C80193 | 3.35 | 1.42 |
| G protein-coupled receptor associated sorting protein 1 (Gprasp1) | Mm.271980 | AW556585 | 3.16 | 1.09 |
| CDC like kinase 4 (Clk4) | Mm.239354 | AW554767 | 3.07 | 1.28 |
| Splicing factor, arginine/serine-rich 6 (Sfrs6) | Mm.24042 | AW559147 | 3.05 | 0.89 |
| Sperm-associated antigen 9 (Spag9) | Mm.260737 | AW553401 | 3.02 | 1.37 |
| RIKEN cDNA C920008N22 gene (Hbxap) | Mm.211743 | AW556115 | 3.02 | 1.09 |
| RNA binding motif protein 18 (Rbm18) | Mm.205937 | C87520 | 2.88 | 1.37 |
| Ubiquilin 1 (Ubqln1) | Mm.182053 | C81478 | 2.75 | 1.08 |
| RIKEN cDNA 2900046G09 gene (2900046G09Rik) | Mm.196512 | AW550265 | 2.72 | 1.17 |
| Chromodomain helicase DNA binding protein 1 (Chd1) | Mm.8137 | AU045087 | 2.66 | 1.09 |
| RIKEN cDNA B930096F20 gene (Stx5a) syntaxin 5a | Mm.153061 | AW555342 | 2.56 | 1.08 |
| Farnesyltransferase, CAAX box, alpha (Fnta) | Mm.3496 | AW556923 | 2.46 | 1.25 |
| DNA segment, Chr2, ERATO Doi 435, expressed (D2Ertd435e) | Mm.283361 | C86136 | 2.24 | 1.26 |
| FUS interacting protein (serine-arginine rich) 1 (Fusip1) | Mm.10229 | AW537256 | 2.21 | 1.14 |
| RNA binding protein gene with multiple splicing (Rbpms) | Mm.323997 | AU019051 | 2.20 | 1.27 |
| Tubulin, gamma 1 (Tubg1) | Mm.142348 | AW539270 | 2.13 | 1.29 |
| Amyloid beta precursor protein (cytoplasmic tail) binding protein 2 (Appbp2) | Mm.271997 | AW544549 | 2.12 | 1.23 |
| Physiological process | ||||
|
| ||||
| Calponin 1 (Cnn1) | Mm.4356 | C86052 | 4.16 | 1.28 |
| CCR4 carbon catabolite repression 4-like (S. cerevisiae) (Ccrn4l) | Mm.86541 | AU043840 | 2.88 | 1.11 |
| Cellular process | ||||
|
| ||||
| G-protein signalling modulator 2 (AGS3-like, C. elegans) (Gpsm2) | Mm.226941 | AW537963 | 11.96 | 1.32 |
| Phosphodiesterase 8A (Pde8a) | Mm.322891 | C87459 | 4.61 | 1.15 |
| Ras association (RalGDS/AF-6) domain family 1 (Rassf1) | Mm.12091 | AU044980 | 2.56 | 1.41 |
| Unknown | ||||
|
| ||||
| DiGeorge syndrome critical region gene 6 (Dgcr6) | Mm.27155 | AU045383 | 10.12 | 1.48 |
| Calmodulin binding transcription activator 1 (Camta1) | Mm.318846 | AU024273 | 6.98 | 1.02 |
| RIKEN cDNA F830004D09 gene (Eml4) | Mm.295565 | AW556280 | 2.51 | 1.27 |
| No function in level 2 | ||||
|
| ||||
| PHD finger protein 17 (Phf17) | Mm.286285 | AW551496 | 8.61 | 1.64 |
| Oocyte specific homeobox 2 (Obox1) | Mm.358932 | AU046150 | 3.85 | 1.34 |
| Ciliary neurotrophic factor receptor (Cntfr) | Mm.272210 | AU018997 | 2.93 | 1.26 |
| Ly1 antibody reactive clone (Lyar) | Mm.28560 | AU044713 | 2.91 | 1.32 |
| Lymphocyte antigen 6 complex, locus G6C (Ly6g6c) | Mm.215096 | AU016360 | 2.74 | 1.07 |
| Glypican 1 (Gpc1) | Mm.297976 | AW555635 | 2.47 | 1.34 |
| Zinc finger, A20 domain containing 2 (Za20d2) | Mm.292405 | AU043297 | 2.40 | 1.27 |
| Mitochondrial tumour suppressor 1 (Mtus1) | Mm.149438 | AW552927 | 2.20 | 0.92 |
| Without GO annotation | ||||
|
| ||||
| RIKEN cDNA 1500041J02 gene (1500041J02Rik) | Mm.281019 | AW538623 | 6.59 | 1.79 |
| MYST histone acetyltransferase monocytic leukaemia 4 (Myst4) | Mm.248967 | C85086 | 5.55 | 0.97 |
| RIKEN cDNA 9130229H14 gene (9130229H14Rik) | Mm.266884 | C80126 | 5.31 | 1.10 |
| RIKEN cDNA 1110003E01 gene (1110003E01Rik) | Mm.10709 | AU023219 | 5.30 | 1.48 |
| Downstream of Stk11 (Dos) | Mm.44231 | C85710 | 4.66 | 1.13 |
| Myocyte enhancer factor 2D (Mef2d) | Mm.28184 | C86932 | 4.55 | 1.14 |
| RIKEN cDNA 1110007L15 gene (1110007L15Rik) | Mm.319134 | AW555473 | 4.42 | 1.31 |
| H3 histone, family 3B LOC433382 | Mm.18516 | AW539780 | 4.13 | 0.76 |
| Fyn proto-oncogene (Fyn) | Mm.4848 | AW552119 | 3.93 | 0.97 |
| RIKEN cDNA 1500011J06 gene (1500011J06Rik) | Mm.276341 | AW557796 | 3.80 | 1.77 |
| Trk-fused gene (Tfg) | Mm.235108 | AU021269 | 3.78 | 1.22 |
| Ring finger protein 139 (Rnf139) | Mm.4537 | AW538451 | 3.74 | 1.42 |
| Growth arrest specific 5 (Gas5) | Mm.270065 | AW546280 | 3.51 | 1.28 |
| RIKEN cDNA 2510001I10 gene (2510001I10Rik) | Mm.29432 | C76468 | 3.47 | 1.19 |
| RIKEN cDNA 5830415L20 gene (5830415L20Rik) | Mm.240265 | AU023795 | 3.43 | 1.15 |
| RIKEN cDNA 2410002M20 gene (2410002M20Rik) | Mm.157534 | AW544376 | 3.23 | 1.27 |
| Wdr45-like (Wdr45l) | Mm.103986 | AW552315 | 3.18 | 0.98 |
| RIKEN cDNA 2810017D21 gene (Pum1) pumilio 1 (Drosophila) | Mm.34701 | AU044062 | 3.14 | 0.93 |
| RIKEN cDNA 5830435K17 gene (5830435K17Rik) | Mm.155687 | AU019572 | 2.91 | 1.25 |
| Protein phosphatase 1, regulatory (inhibitor) subunit 12A (Ppp1r12a) | Mm.207499 | AU015467 | 2.77 | 1.27 |
| Enhancer of polycomb homologue 2 (Drosophila) (Epc2) | Mm.29167 | AU040241 | 2.69 | 0.93 |
| RIKEN cDNA 1110067M05 gene (1110067M05Rik) | Mm.341886 | AU015454 | 2.55 | 1.06 |
| RIKEN cDNA 4121402D02 gene (Casc3) | Mm.40120 | AW556296 | 2.54 | 1.13 |
| RIKEN cDNA A730098D12 gene (A730098D12Rik) | Mm.196325 | C86491 | 2.53 | 1.10 |
| RIKEN cDNA 1700037H04 gene (1700037H04Rik) | Mm.27711 | AU041152 | 2.49 | 0.99 |
| WD repeat domain 26 (Wdr26) | Mm.289082 | AW540967 | 2.47 | 0.94 |
| Eukaryotic translation termination factor 1 (Etf1) | Mm.329353 | AU016000 | 2.42 | 1.75 |
|
DNA segment, Chr 1, Brigham & Women’s Genetics 1363 expressed
(D1Bwg1363e) |
Mm.260577 | AW553790 | 2.41 | 1.08 |
| Plakophilin 4 (Pkp4) | Mm.260938 | AW549051 | 2.39 | 0.84 |
| Muscleblind-like 2 (Mbnl2) | Mm.238266 | AU041504 | 2.28 | 1.00 |
| RIKEN cDNA 9630050M13 gene (9630050M13Rik) | Mm.23044 | AA409679 | 2.23 | 1.27 |
| RIKENcDNA 3321401G04 gene (3321401G04Rik) | Mm.24652 | AW558113 | 2.16 | 1.65 |
Classification was performed according to the GeneOntology biological process level 2 reference.
Functional classification of the genes activated by PACAP plus cycloheximide
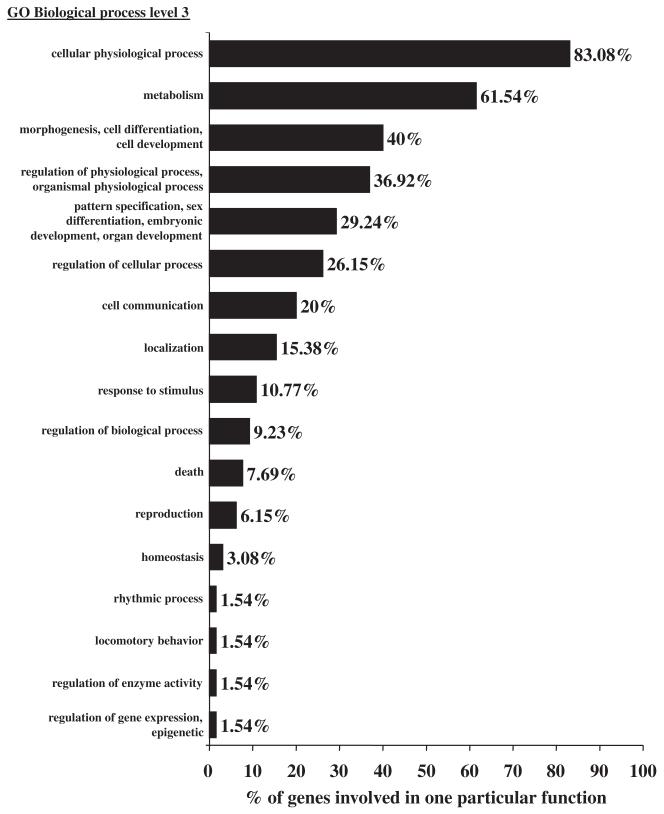
To facilitate further analysis, the genes identified in the present study were classified according to the GeneOntology (GO) references (http://www.geneontology.org/) using the Unigene accession number provided for each clone (Fig. 2, Table 2). Among the 106 mRNA regulated by PACAP plus cycloheximide but not by PACAP alone after 6 h of treatment, 63 genes had an inferred function and were assigned to 17 categories as indexed in the biological process level three of the GO database (Fig. 2). The three most abundantly populated categories were, in descending order: cellular physiological processes, metabolism and morphogenesis, and cell differentiation and development. As PACAP can either promote cell differentiation or inhibit apoptosis, it is interesting to note in Table 2 the presence of several genes controlling growth arrest, i.e. growth arrest and DNA-damage-inducible 45 gamma (Gadd45g), morphogenesis/cell differentiation, i.e. signal transducer and activator of transcription 3 (Stat3), embryonic development, i.e. ring finger protein 2 (Rnf2) or cell death, i.e. Sgk.
Fig. 2.
Functional classification according to the GeneOntology (GO) reference of the mRNA activated by pituitary adenylate cyclase-activating polypeptide (PACAP) plus cycloheximide (biological process level three). Cluster analysis was conducted with the FatiGO application (http://www.fatigo.org). As illustrated with FBJ osteosarcoma-related oncogene (Fos), which belongs to six categories indexed in GO biological process level three (pattern specification, sex differentiation, embryonic development and organ development; metabolism; morphogenesis, cell differentiation and cell development; regulation of physiological process, organismal physiological process; regulation of cellular process and finally cellular physiological process), one particular gene can be assigned to several GO annotations.
Three genes have no known role in the GO and eight have an identified function but are not present in the biological process level two categories (Table 2). Finally, 32 genes regulated by PACAP plus cycloheximide (30%) were not referenced in the GO database. A majority of these genes code for RIKEN cDNA (50%), and the others for genes that have not yet been entered in the database. Some of the transcripts without GO annotation, such as growth arrest specific 5 (Gas5), are nevertheless likely candidates for controlling aspects of the neurotrophic effects of PACAP.
Verification of gene induction by real-time PCR
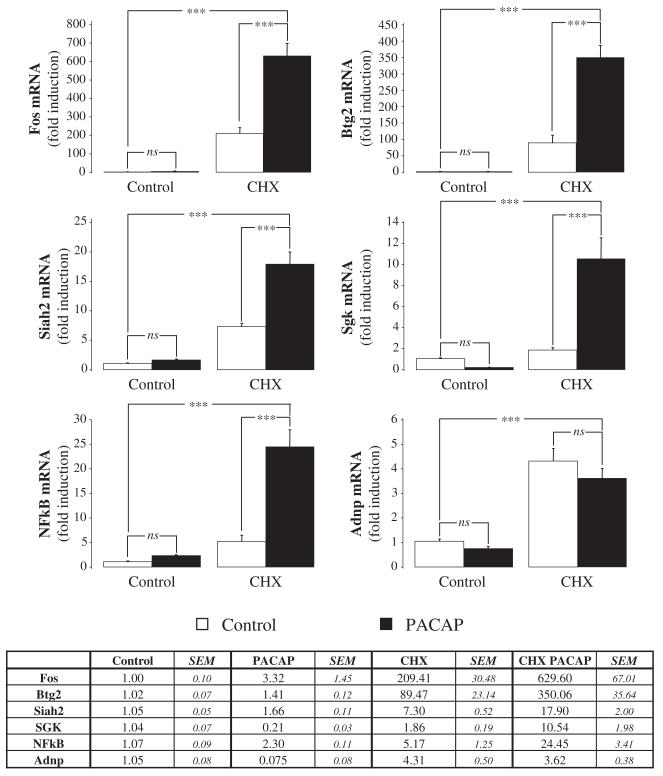
Six genes with varying expression profiles were selected and their expression level was quantified by real-time PCR to validate the microarray results (Table 1). Primer efficacy was addressed by measuring the slope of a standard curve taken from a serial dilution of control cDNA using the glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA as an internal standard. The real-time PCR results confirmed the microarray data as all the genes tested by quantitative PCR were indeed significantly induced after 6 h of treatment with PACAP plus cycloheximide (Fig. 3). Real-time PCR experiments also revealed that nuclear factor for κ chain of B-cells (NFκB) and Fos were induced to 2.3- and 3.3-fold levels, respectively, by PACAP alone (Fig. 3), but this was not significant according to our statistical analysis. Incubation of the cells with cycloheximide alone increased the expression level of the genes tested from 1.9- (Sgk) to 209-fold (Fos) (Fig. 3) and, for five genes out of six, the addition of PACAP resulted in a synergistic and highly significant increase in transcript levels. Maximum expression was observed for Fos, which was induced to a level of 630-fold higher than the control level after 6 h of exposure to PACAP plus cycloheximide. The only gene not activated by PACAP in the presence of cycloheximide was the activity-dependent neuroprotective protein (Adnp). Adnp is known to be regulated by PACAP, but this gene seems to be induced through the VPAC2 receptor which is not expressed in PC12 cells (Zusev and Gozes 2004).
Fig. 3.
Independent verification of representative genes regulated by pituitary adenylate cyclase-activating polypeptide (PACAP) in PC12 cells. Quantification of FBJ osteosarcoma-related oncogene (Fos), β-cell translocation gene 2, antiproliferative (Btg2), seven in absentia 2 (Siah2), serum/glucocorticoid-regulated kinase (Sgk), ubiquitin-conjugating enzyme E2D 3 (UBC4/5 homologue, yeast) (NFκB) and activity-dependent neuroprotective protein (Adnp) expression levels by real-time PCR after 6 h of treatment with either PACAP (10−7 m) alone or in the presence of cycloheximide (5 μm). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA was used as an internal standard. ***p < 0.001; ns, not statistically different.
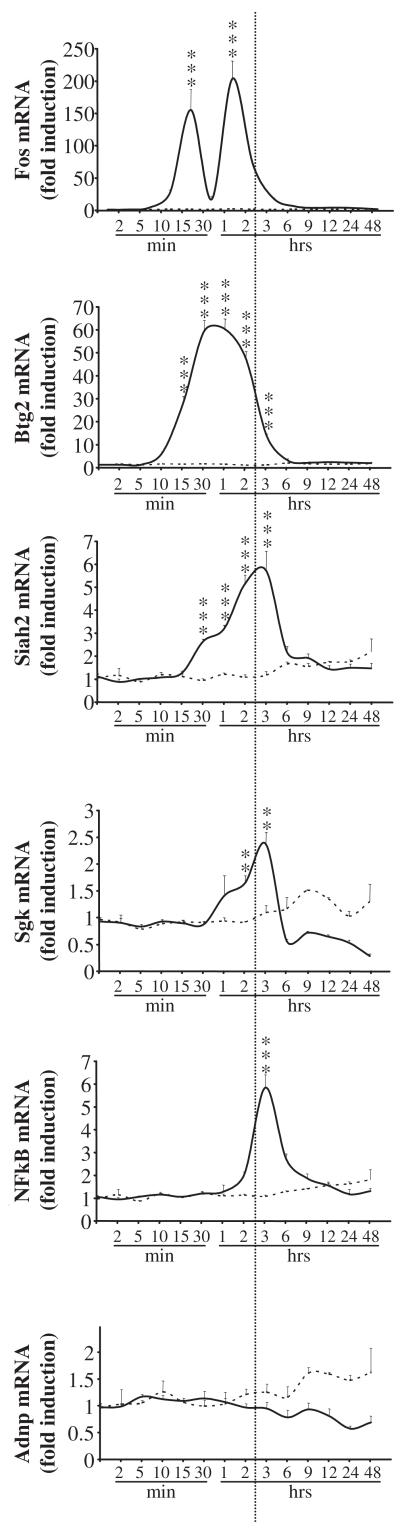
The possible effect of PACAP alone on the expression of the six genes tested by real-time PCR was investigated by exposure to PACAP (10−7 m) for durations ranging from 2 min to 6 h, with additional time points of 12, 24 and 48 h measured after the first 6-h period (Fig. 4). This time-course experiment revealed that all the genes activated by PACAP in the presence of cycloheximide, with the exception of Adnp were also induced by PACAP alone. The activation of some transcripts such as Fos and Btg2 occurred within 10 mins, whereas it took 2 h for NFκB to be induced. Maximum expression observed after 1–3 h of treatment with PACAP was only 2.5 over control for Sgk, and exceeded 200-fold over control for Fos (Fig. 4). After 6 h of treatment, all transcripts in this group returned to basal levels of expression.
Fig. 4.
Time-course of pituitary adenylate cyclase-activating polypeptide (PACAP) effect on gene expression. PC12 cells were exposed to PACAP (10−7 m) for durations ranging from 2 min to 48 h and the gene-expression profile was measured by real-time PCR for FBJ osteosarcoma-related oncogene (Fos), β-cell translocation gene 2, antiproliferative (Btg2), seven in absentia 2 (Siah2), serum/glucocorticoid-regulated kinase (Sgk), nuclear factor for kappa chain of B-cells (NFκB) and activity-dependent neuroprotective protein (Adnp). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA was used as an internal standard. Solid lines correspond to the mRNA expression in PACAP-treated cells and dashed lines correspond to the mRNA expression in cells treated with control medium. **p < 0.01; ***p < 0.001; unmarked data points, not statistically different from control.
Discussion
Recent studies performed on PC12 cells after 0.5, 6 and 48 h revealed that several populations of early as well as late-response genes are induced by PACAP after various times of treatment, highlighting the difficulty of selecting a single and optimal time point for microarray experiments (Vaudry et al. 2002a; Grumolato et al. 2003; Ishido and Masuo 2004). In a previously published study (Vaudry et al. 2002a) we chose a time point of 6 h to examine the effect of PACAP on PC12 cell transcriptome because the removal of PACAP before 6 h resulted in a reduced neuritogenic response at the subsequent assessment at 48 h. The withdrawal of PACAP at 6 h produced the same response at 48 h as that seen with PACAP present during the entire 48-h period. This strongly suggests that PACAP triggers the differentiation program by, but not before, 6 h of treatment, which 48 h later results in neurite extension, cessation of cell proliferation and the production of a battery of transcripts required for full expression of the neuroendocrine phenotype. The design of our previous study overlooked, however, those transcripts transiently contributing to the regulatory cascade leading to full differentiation. So-called immediate early genes, for example, are likely to be found within the cohort of genes making up the transitional transcriptome. Immediate early genes are defined as those which encode proteins that act mainly as transacting factors for transcription, in order to regulate either the expression of the genes encoding the final protein effectors of the cellular processes under investigation (Morgan and Curran 1995).
The present study offers a useful approach to identify genes regulated in a transient manner that may not be detected at a given single time point, which most microarray studies are restricted to for practical considerations. The real-time PCR experiments indicated that PACAP can activate some genes such as Btg2 within 10 min of treatment. PCR validation conducted on six different genes also confirmed the microarray results and revealed that five transcripts out of six were actually also regulated by PACAP alone. This observation indicates that most of the genes presented in Table 2 are likely to be induced by PACAP alone as part of the transitional transcriptome(s) leading to PC12 cell differentiation. As the regulation of all genes of interest can be easily validated by real-time PCR, as illustrated for the six transcripts examined here, the important question will now be the identification of the function of each of these genes during PC12 cell differentiation, and specifically either their role in the induction of downstream targets at the transcriptional level or the enhancement of effector function through transient effects on phosphorylation, proteolysis or other modes of protein activation.
Effects of PACAP on transcription factors
The functional classification performed with the GO database highlighted 15 transcription factors known to be regulated rapidly and transiently. Some of these messengers encoding CREB binding protein (Crebbp) and Stat3 have already been shown to regulate the expression of other late response genes such as either tyrosine hydroxylase or neuropeptide Y in PC12 cells (Ghee et al. 1998; Muraoka et al. 2003). Conversely, some genes, which have previously been shown to be strongly induced by PACAP after 6 h of treatment, such as the immediate early response 3 (Ier3), contain in their promoter region functional binding sites for NFκB (Schafer et al. 1998; Vaudry et al. 2002a). As this transcription factor is activated within 2 h of treatment with PACAP it may participate in the induction of Ier3. NFκB is usually associated with either Tumor Necrosis Factor-α (TNF-α) or Fas ligand-induced apoptosis, but it has also been demonstrated that inhibition of this transcription factor by sodium salicylate leads to PC12 cell death (Kiss et al. 2004). In addition, NFκB inhibitors exacerbate oxidative stress-induced cell death and play a role in B-cell lymphoma protein 2 (Bcl-2) protection (Jang and Surh 2004). These observations suggest that the genes regulated through NFκB may potentially control the anti-apoptotic effects of PACAP.
Some of the early response genes regulated by PACAP are likely to convey its neurotrophic effects in PC12 cells (Tanaka et al. 1997). For instance, Fos, previously identified as transiently up-regulated by PACAP in cultured cerebellar granule neurones (Vaudry et al. 2000), was shown to be strongly induced by cycloheximide plus PACAP but also by PACAP alone in PC12 cells. The involvement of Fos in the neurotrophic effects of PACAP is supported by the fact that blocking Fos expression in PC12 cells inhibits the differentiation induced by NGF (Gil et al. 2004). Jumonji (Jarid 2), another gene regulated by PACAP in the presence of cycloheximide, encodes a protein required for proper neural tube formation (Takeuchi et al. 1995) and the reduction of cardiac cell proliferation via the repression of cyclin D1 expression (Toyoda et al. 2003). Based on this information, Jumonji is a likely candidate for involvement in the effects of PACAP on either cell proliferation or neuritogenesis, and work is in progress to test this hypothesis directly through the abrogation of Jumonji induction during PACAP-induced neuronal differentiation of PC12 cells.
Treatment with PACAP plus cycloheximide also increased the transcript for activating transcription factor 3 (Atf3), a protein that can stimulate cell proliferation (Tamura et al. 2005), enhance cell survival (Francis et al. 2004) or promote apoptosis (Hai and Hartman 2001) after exposure to stress agents. The variability in the effects of this protein, which is usually increased when cells are exposed to stress signals such as ischemia, alcohol, UV radiation or axotomy, could be a result of the fact that the ATF3 homodimer is a transcriptional repressor, whereas heterodimeric association with Jun proteins produces a transcriptional activator (Hai and Hartman 2001). In particular, overexpression of ATF3 in c-Jun-activated PC12 cells promotes heat shock protein 27 (Hsp27) expression (Benn et al. 2002), which in turn rescues cell survival and induces neurite outgrowth (Nakagomi et al. 2003). Co-expression of Atf3 with c-Jun significantly enhances c-Jun-mediated neurite sprouting, suggesting that these two transcription factors initiate an axonal regeneration program in response to axotomy (Pearson et al. 2003). Altogether these results suggest that Atf3 could mediate some of the neurotrophic effects of PACAP in PC12 cells, and the possible regulation of Atf3 by PACAP is also supported by the fact that this protein belongs to the ATF/CREB family of transcription factors known to be induced via the cAMP pathway (Gao et al. 2004).
Genes potentially involved in cell neuroprotection and differentiation
PACAP has been shown to reduce PC12 cell proliferation, promote neurite outgrowth and inhibit apoptosis (Vaudry et al. 2002b). It was thus interesting to classify the genes according to their putative function in order to identify those potentially involved in the neurotrophic effects of PACAP. Apart from the genes involved in the control of mRNA transcription, a significant proportion of other messengers could also control either apoptosis or cell proliferation. In particular, it has been shown that Btg2, which is induced by more than 60-fold by PACAP, inhibits cell proliferation when overexpressed in either NIH3T3 or PC12 cells (Duriez et al. 2004). In vivo overexpression of Btg2 inhibits cyclin D1 expression and increases the synthesis of the transcription factor Math1, which is required for neurogenesis (Canzoniere et al. 2004). Finally, it has been reported that in PC12 cells Btg2 potentiates NGF-induced differentiation and protects these cells from apoptosis elicited by NGF deprivation (Corrente et al. 2002). As in PC12 cells both PACAP and NGF initiate differentiation and inhibit apoptosis (Vaudry et al. 2002b), Btg2 is likely to have the same role when activated by PACAP as by NGF.
In the presence of cycloheximide, PACAP also activated Sgk, to a level of more than 10-fold, which has been reported to inhibit apoptosis of human (Mikosz et al. 2001) and rat mammary tumour cells (Webster et al. 1993). Besides its neuroprotective effect Sgk also promotes dendrite outgrowth in neurones (David et al. 2005), thereby supporting the possible involvement of this protein kinase in the neurotrophic effects of PACAP on PC12 cells.
Perhaps the most intriguing of the transiently regulated transcripts discovered here is Siah2. The structurally related Siah1 has recently been strongly implicated in NO-mediated apoptotic signalling through Gapdh in PC12 cells upon neurotrophin withdrawal and macrophage cell death following exposure to lipopolysaccharides (LPS) (Hara et al. 2005), and may be involved in Gapdh-dependent cerebellar granule cell death during development (Ishitani et al. 1996; Hara and Snyder 2006). Whether Siah2 modulates this pathway or participates in a parallel cell-survival pathway initiated by exposure to PACAP, remains to be investigated.
In conclusion, the present report has identified by microarray about one hundred genes activated by PACAP in the presence of cycloheximide that had not previously been shown to be regulated by PACAP alone. The real-time PCR experiments have revealed that a significant proportion of these genes is transiently induced by PACAP alone. To the best of our knowledge, this is the first study using this approach to characterize early response genes regulated by neuropeptides. Some of the proteins encoded by mRNA identified in the present study are likely to participate in the effects of PACAP on PC12 cell differentiation. Further analysis of the transduction pathways regulating these genes, investigation of their functional significance in PC12 cells, and the determination of their role in other cell types will provide a better understanding of the mechanisms involved in the neurotrophic effects of PACAP during development and in pathological conditions.
Acknowledgements
This work was supported by INSERM (U413), the European Institute for Peptide Research (IFRMP23), the National Institute of Mental Health (NIMH) Intramural Research Program, the Association pour la Recherche sur le Cancer and the Conseil Régional de Haute-Normandie. AR was the recipient of a doctoral fellowship from the Ministry of Education.
Abbreviations used
- Adnp
activity-dependent neuroprotective protein
- Arc
activity-regulated cytoskeleton-associated protein
- Atf3
activating transcription factor 3
- Btg2
β-cell translocation gene 2, antiproliferative
- Cited1
Cbp/p300-interacting transactivator with Glu/Asp-rich carboxterminal domain 1
- Crebbp
CREB binding protein
- Egr1
early growth response 1
- Fos
FBJ osteosarcoma related oncogene
- Gadd45g
growth arrest and DNA-damage-inducible 45 gamma
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- Gas5
growth arrest specific 5
- GO
GeneOntology
- Hsp27
heat shock protein 27
- Ier3
immediate early response 3
- NFκB
nuclear factor for the κ chain of B-cells
- NGF
nerve growth factor
- PACAP
pituitary adenylate cyclase-activating polypeptide
- Rnf2
ring finger protein 2
- SDS
sodium dodecyl sulfate
- Sgk
serum/glucocorticoid-regulated kinase
- Siah2
seven in absentia homologue 2
- SSC
saline sodium citrate
- Stat3
signal transducer and activator of transcription 3
- 3′UTR
3′ untranslated regions
References
- Basille M, Vaudry D, Coulouarn Y, Jégou S, Lihrmann I, Fournier A, Vaudry H, Gonzalez BJ. Comparative distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) binding sites and PACAP receptor mRNAS in the rat brain during development. J. Comp. Neurol. 2000;425:495–509. [PubMed] [Google Scholar]
- Benn SC, Perrelet D, Kato AC, Scholz J, Decosterd I, Mannion RJ, Bakowska JC, Woolf CJ. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36:45–56. doi: 10.1016/s0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- Canzoniere D, Farioli-Vecchioli S, Conti F, et al. Dual control of neurogenesis by PC3 through cell cycle inhibition and induction of Math1. J. Neurosci. 2004;24:3355–3369. doi: 10.1523/JNEUROSCI.3860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc. Natl Acad. Sci. USA. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrente G, Guardavaccaro D, Tirone F. PC3 potentiates NGF-induced differentiation and protects neurons from apoptosis. Neuroreport. 2002;13:417–422. doi: 10.1097/00001756-200203250-00011. [DOI] [PubMed] [Google Scholar]
- David S, Stegenga SL, Hu P, Xiong G, Kerr E, Becker KB, Venkatapathy S, Warrington JA, Kalb RG. Expression of serum- and glucocorticoid-inducible kinase is regulated in an experience-dependent manner and can cause dendrite growth. J. Neurosci. 2005;25:7048–7053. doi: 10.1523/JNEUROSCI.0006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch PJ, Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J. Biol. Chem. 1992;267:5108–5113. [PubMed] [Google Scholar]
- Duriez C, Moyret-Lalle C, Falette N, El-Ghissassi F, Puisieux A. BTG2, its family and its tutor. Bull. Cancer. 2004;91:E242–E253. [PubMed] [Google Scholar]
- Edwards DR, Mahadevan LC. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms: lack of evidence for labile repressors. EMBO J. 1992;11:2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis JS, Dragunow M, During MJ. Over expression of ATF-3 protects rat hippocampal neurons from in vivo injection of kainic acid. Mol. Brain Res. 2004;124:199–203. doi: 10.1016/j.molbrainres.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Gao MH, Tang T, Guo T, Sun SQ, Feramisco JR, Hammond HK. Adenylyl cyclase type VI gene transfer reduces phospholamban expression in cardiac myocytes via activating transcription factor 3. J. Biol. Chem. 2004;279(38):797–38. 802. doi: 10.1074/jbc.M405701200. [DOI] [PubMed] [Google Scholar]
- Ghee M, Baker H, Miller JC, Ziff EB. AP-1, CREB and CBP transcription factors differentially regulate the tyrosine hydroxylase gene. Mol. Brain Res. 1998;55:101–114. doi: 10.1016/s0169-328x(97)00370-7. [DOI] [PubMed] [Google Scholar]
- Gil GA, Bussolino DF, Portal MM, Pecchio AA, Renner ML, Borioli GA, Guido ME, Caputto BL. c-Fos activated phospholipid synthesis is required for neurite elongation in differentiating PC12 cells. Mol. Biol. Cell. 2004;15:1881–1894. doi: 10.1091/mbc.E03-09-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl Acad. Sci. USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumolato L, Elkahloun AG, Ghzili H, et al. Microarray and suppression subtractive hybridization analyses of gene expression in pheochromocytoma cells reveal pleiotropic effects of pituitary adenylate cyclase-activating polypeptide on cell proliferation, survival, and adhesion. Endocrinology. 2003;144:2368–2379. doi: 10.1210/en.2002-0106. [DOI] [PubMed] [Google Scholar]
- Hai T, Hartman MG. The molecular biology and nomen-clature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- Hara MR, Agrawal N, Kim SF, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- Hara MR, Snyder SH. Nitric oxide-GAPDH-Siah: A and novel cell death cascade. Cell. Mol. Neurobiol. 2006 doi: 10.1007/s10571-006-9011-6. in press, doi: 10.1007/s10571-006-9011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H, Fujimoto T, Taira E, Miki N. The accumulation of arc (an immediate early gene) mRNA by the inhibition of protein synthesis. J. Pharmacol. Sci. 2003;91:247–254. doi: 10.1254/jphs.91.247. [DOI] [PubMed] [Google Scholar]
- Ishido M, Masuo Y. Transcriptome of pituitary adenylate cyclase-activating polypeptide-differentiated PC12 cells. Regul. Pept. 2004;123:15–21. doi: 10.1016/j.regpep.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Ishitani R, Sunaga K, Hirano A, Saunders P, Katsube N, Chuang DM. Evidence that glyceraldehyde-3-phosphate dehydrogenase is inovlved in age-induced apoptosis in mature cerebellar neurons in culture. J. Neurochem. 1996;66:928–935. doi: 10.1046/j.1471-4159.1996.66030928.x. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Bcl-2 attenuation of oxidative cell death is associated with up-regulation of gamma-glutamylcysteine ligase via constitutive NF-kappaB activation. J. Biol. Chem. 2004;279(38):779–38. 786. doi: 10.1074/jbc.M406371200. [DOI] [PubMed] [Google Scholar]
- Kiss K, Kiss J, Rudolf E, Cervinka M, Szeberenyi J. Sodium salicylate inhibits NF-kappaB and induces apoptosis in PC12 cells. J. Biochem. Biophys. Meth. 2004;61:229–240. doi: 10.1016/j.jbbm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J. Biol. Chem. 2001;276(16):649–16. 654. doi: 10.1074/jbc.M010842200. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Immediate-early genes: ten years on. Trends Neurosci. 1995;18:66–67. [PubMed] [Google Scholar]
- Muraoka O, Xu B, Tsurumaki T, Akira S, Yamaguchi T, Higuchi H. Leptin-induced transactivation of NPY gene promoter mediated by JAK1, JAK2 and STAT3 in the neural cell lines. Neurochem. Int. 2003;42:591–601. doi: 10.1016/s0197-0186(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Nakagomi S, Suzuki Y, Namikawa K, Kiryu-Seo S, Kiyama H. Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J. Neurosci. 2003;23:5187–5196. doi: 10.1523/JNEUROSCI.23-12-05187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson AG, Gray CW, Pearson JF, Greenwood JM, During MJ, Dragunow M. ATF3 enhances c-Jun – mediated neurite sprouting. Mol. Brain Res. 2003;120:38–45. doi: 10.1016/j.molbrainres.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Roshak A, Jackson JR, McGough K, Chabot-Fletcher M, Mochan E, Marshall LA. Manipulation of distinct NFκB proteins alters interleukin-1α-induced human rheumatoid synovial fibroblast prostaglandin E2 formation. J. Biol. Chem. 1996;271(31):496–31. 501. doi: 10.1074/jbc.271.49.31496. [DOI] [PubMed] [Google Scholar]
- Schafer H, Diebel J, Arlt A, Trauzold A, Schmidt WE. The promoter of human p22/PACAP response gene 1 (PRG1) contains functional binding sites for the p53 tumor suppressor and for NFkappaB. FEBS Lett. 1998;436:139–143. doi: 10.1016/s0014-5793(98)01109-0. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Yamazaki Y, Katoh-Fukui Y, Tsuchiya R, Kondo S, Motoyama J, Higashinakagawa T. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 1995;9:1211–1222. doi: 10.1101/gad.9.10.1211. [DOI] [PubMed] [Google Scholar]
- Tamura K, Hua B, Adachi S, et al. Stress response gene ATF3 is a target of c-myc in serum-induced cell proliferation. EMBO J. 2005;24:2590–2601. doi: 10.1038/sj.emboj.7600742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Koshimura K, Murakami Y, Sohmiya M, Yanaihara N, Kato Y. Neuronal protection from apoptosis by pituitary adenylate cyclase-activating polypeptide. Regul. Pept. 1997;72:1–8. doi: 10.1016/s0167-0115(97)01038-0. [DOI] [PubMed] [Google Scholar]
- Tanaka TS, Jaradat SA, Lim MK, et al. Genome-wide expression profiling of mid-gestation placenta and embryo using a 15,000 mouse developmental cDNA microarray. Proc. Natl Acad. Sci. USA. 2000;97:9127–9132. doi: 10.1073/pnas.97.16.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda M, Shirato H, Nakajima K, Kojima M, Takahashi M, Kubota M, Suzuki-Migishima R, Motegi Y, Yokoyama M, Takeuchi T. jumonji downregulates cardiac cell proliferation by repressing cyclin D1 expression. Dev. Cell. 2003;5:85–97. doi: 10.1016/s1534-5807(03)00189-8. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Anouar Y, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide stimulates both c-fos gene expression and cell survival in rat cerebellar granule neurons through activation of the protein kinase A pathway. Neuroscience. 1998;84:801–812. doi: 10.1016/s0306-4522(97)00545-9. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Vaudry D, Chen Y, Ravni A, Hamelink C, Elkahloun AG, Eiden LE. Analysis of the PC12 cell transcriptome after differentiation with pituitary adenylate cyclase-activating polypeptide (PACAP) J. Neurochem. 2002a;83:1272–1284. doi: 10.1046/j.1471-4159.2002.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002b;296:1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein FO, Noll H, Penman S. Effect of cycloheximide on ribosomal aggregates engaged in protein synthesis in vitro. Biochim. Biophys. Acta. 1964;87:525–528. doi: 10.1016/0926-6550(64)90131-8. [DOI] [PubMed] [Google Scholar]
- Zusev M, Gozes I. Differential regulation of activity-dependent neuroprotective protein in rat astrocytes by VIP and PACAP. Regul. Pept. 2004;123:33–41. doi: 10.1016/j.regpep.2004.05.021. [DOI] [PubMed] [Google Scholar]