Abstract
In recent years the scientific community has generated an ever-increasing amount of data from a growing number of animal models of human cancers. Much of these data come from genetically engineered mouse models. Identifying appropriate models for skin cancer and related relevant genetic data sets from an expanding pool of widely disseminated data can be a daunting task. The Mouse Tumor Biology Database (MTB) provides an electronic archive, search, and analysis system that can be used to identify dermatological mouse models of cancer, retrieve model-specific data, and analyze these data. In this report we detail MTB’s contents and capabilities, together with instructions on how to use MTB to search for skin-related tumor models and associated data.
Keywords: animal model, skin cancer, tumor, mouse, pathology, database, images
Background
The explosion in the amount and complexity of data generated by molecular biology and genomics technologies has made traditional approaches to the identification and analysis of relevant data difficult. Large-scale data generating projects, such as the human genome sequencing project (1), the ENCODE project (2), and Genome Wide Association Studies (GWAS) (3) exemplify this problem. Virtually all fields of biological study face this data deluge, including dermatology.
Human disease model systems have assumed an important role in the production of phenotypic and genetic dermatology related information. The laboratory mouse has been and continues to be the most commonly used and important model system used in studying human skin. However, while important, the utility of mouse models is limited by the complexity of the disease and the inbred nature of the mouse genetics (4, 5). Mouse models are widely used because mice are easily maintained, have a fully sequenced and annotated genome, can be studied at all life stages even before disease onset, and have an extensive array of molecular and genetic tools available to manipulate their pathophysiology (6–8). Mouse models are particularly advantageous when studying human diseases due to their similar physiology, and numerous established inbred, congenic, consomic, and recombinant inbred strains. Additionally, patient-derived xenograft (PDX) models based on human tumor grafts grown in mouse hosts are becoming more important in translational research (9).
Identifying and accessing skin-related mouse model data from the published literature and sifting through the strains, mutations, nomenclature, and pathological descriptions generated from these models to enable new discoveries and facilitate experimental designs is no longer feasible for an individual working manually on this corpus.
Questions Addressed
The ability to locate very specific dermatology data related to particular genetic and biological details such as tissue type, related genes, and tumor type is very important in designing future experiments and interpreting current publications. As an example of this, recent dermatology publications use mouse models designed to investigate tumor metastases and lesion staging in transgenic mice and human xenograft models (10–12). How can MTB assist scientists in identifying potentially useful and highly specific mouse models of dermatological tumors and access and analyze the diverse amount of data associated with these models?
Experimental Design
MTB has been publicly available since 1998 (13–15). MTB was constructed with the goal of making available a central electronic resource for the collection, integration, and analysis of the diverse data derived from mouse cancer models and to support development of new models. MTB incorporates information on the frequency/incidence, latency, and tissue of origin of mouse tumors and metastases. Detailed pathology reports, and associated images are also available. In addition, MTB includes data on the genetics of the background strain and somatic mutations in the tumors including Spectral Karyotyping (SKY), Comparative Genome Hybridization (CGH), Quantitative Trait Loci (QTL) associated with cancers, and indexes gene expression array data for mouse tumors from the Gene Expression Omnibus (GEO) and the Array Express (16–19). All data are attributed to the original source. MTB enables integrated searches of data from diverse sources through application of multiple controlled vocabularies and standardized nomenclature.
MTB has also developed web forms to access data from the Patent Derived Xenograft (PDX) resource at The Jackson Laboratory (20). This program establishes models of human tumors utilizing the NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice as the host for human tumors (NSG, JR#5557, The Jackson Laboratory). The PDX resource includes comprehensive genomic characterization of engrafted tumors with links to de-identified patient clinical data. The PDX resource currently contains over 320 models from 28 different tissues and 66 tumor types including nine skin tumor (melanoma and squamous cell carcinoma) models.
Going forward, MTB plans to improve access to translational and pre-cilinical data as well as mouse model credentialing data and mouse-human comparative genomics.
Results
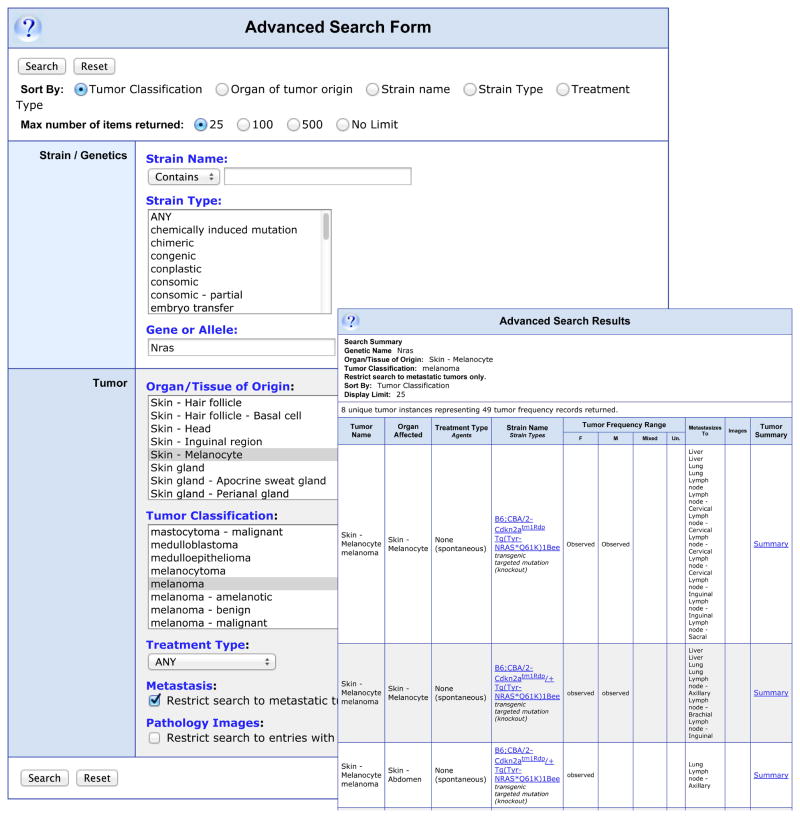
MTB provides a mechanism for researchers to search for dermatology related data in a very detailed manner. For example, to search for data about mouse melanoma models involving Nras that have metastases, users would select the “Advanced Search Form” listed on the left side of the MTB home page. On this form melanoma would be selected from the pull-down list of tumor types and skin-melanocyte as organ of origin. Nras would be entered in the “Gene or Allele” textbox and the “Restrict search to metastatic tumors only.” box would be checked. This search returns 8 “Tumor Instances” (Fig. 1) and shows relevant data for all the search results. The “Summary” links on the right open a detailed summary of the selected tumor and associated metastases with links to References, Pathology Records, and any additional notes. Fig. S1 shows a Pathology Record of an unpublished image of a novel mouse melanoma submitted to MTB by SS Dadras (21).
Figure 1.
Example of a search for metastatic melanoma involving Nras in skin melanocytes. This illustrates the ability of the “Advanced Search Form” to design very detailed queries.
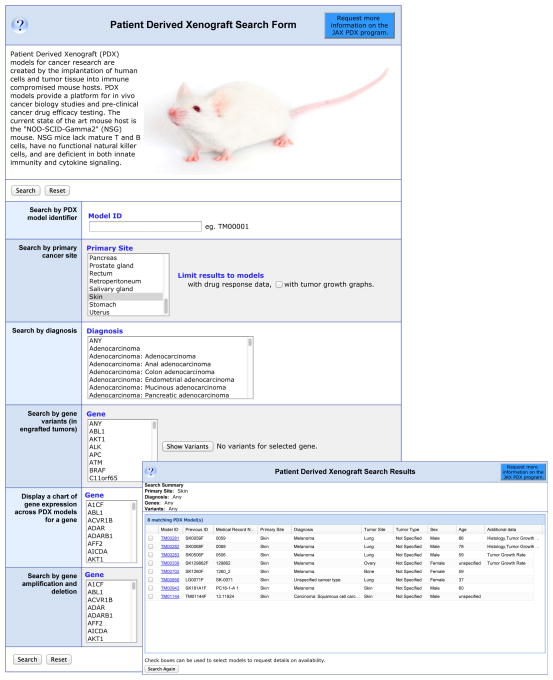
The “Patient Derived Xenograft Search Form” allows researchers to query MTB for PDX models. The search form can be queried by PDX model identifier, primary cancer site, Gene variant, or Gene expression. For example selecting “skin” for primary cancer site returns 8 results: 6 melanomas, 1 squamous cell carcinoma, and an unspecified cancer type. The results also indicate tumor site, patient gender, and age as well as if any additional data, histology, genomics, etc., as shown in Fig. 2. Fig. S2 shows a PDX model details page with histology and tumor growth data.
Figure 2.
Example of a Search for PDX Data. The “Patient Derived Xenograft Search Form” is displayed with “Skin” selected as “Primary Tumor Site”. The inset shows the results of this search.
Conclusions
MTB provides the most comprehensive source of mouse tumor data available, the highest quality of data curation, and search forms that allow data queries from many different scientific perspectives. MTB also serves as a repository for data from the dermatopathology community; for example, including images from the Jackson Aging Center, Pathbase (22, 23), and information on antibodies used to study mouse cancer models. MTB enables researchers to present their tumor data to the scientific community in a way that will place these data in the wider context of genetic and molecular data and assists researchers in identifying appropriate mouse models for their research. Finally, MTB provides community access to key pathology data connected to tumor diagnoses and outcomes that are otherwise unavailable for scientific analysis.
Supplementary Material
Figure S1. “Pathology Image Record” of a tail-skin, transplantable melanoma from a LT.B6 mouse. This “Pathology Record”, in addition to displaying the histopathology image and associated clinical data, also shows the tumor, reference, and strain details.
Figure S2. Example of “Histology” and associated data for a skin melanoma PDX model.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-CA089713, R21-AR063781, and P30-CA034196 (core facilities)).
Begley D.A. aquired the data, and wrote the paper.
Krupke D.M. designed the database, aquired the data, and critically revised the paper.
Neuhauser S.B. wrote the software for the database.
Richardson J.E. designed the software for the database.
Schofield P.N. submitted data, and reviewed the paper.
Bult C.J. designed the database, critically revised the paper.
Eppig J.T. designed the database, critically revised the paper.
Sundberg J.P. submitted data, and wrote the paper.
Footnotes
Conflict of Interest: Dr. Sundberg gave a lecture at the Merz Pharmaceutical Co. within the last 12 months which had no relevance to this paper.
References
- 1.Collins FS, Greene ED, Guttmacher AE, et al. Nature. 2003;422:835–847. doi: 10.1038/nature01626. [DOI] [PubMed] [Google Scholar]
- 2.The ENCODE Project Consortium. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manolio TA. N Engl J Med. 2010;363:166–176. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 4.McGonigle P, Ruggeri B. Biochem Pharmacol. 2014;87:162–171. doi: 10.1016/j.bcp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Ruggeri BA, Camp F, Miknyoczki S. Biochem Pharmacol. 2014;87:150–161. doi: 10.1016/j.bcp.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Carmona-Mora P, Molina J, Encina CA, et al. Curr Genomics. 2009;10:259–68. doi: 10.2174/138920209788488508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaible LM, Corat MA, Abdelhay E, et al. Genet Mol Res. 2010;9:1469–82. doi: 10.4238/vol9-3gmr867. [DOI] [PubMed] [Google Scholar]
- 8.Walrath JC, Hawes JJ, Van Dyke T, et al. Adv Cancer Res. 2010;106:113–64. doi: 10.1016/S0065-230X(10)06004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bankert RB, Balu-Lyer SV, Odunsi K, et al. PLoS One. 2011;6:e24420. doi: 10.1371/journal.pone.0024420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Fits L, Rebel HG, Out-Luiting JJ, et al. Exp Dermatol. 2012;21:706–9. doi: 10.1111/j.1600-0625.2012.01556.x. [DOI] [PubMed] [Google Scholar]
- 11.Schiffner S, Chen S, Becker JC, Bosserhoff AK. Exp Dermatol. 2012;21:786–8. doi: 10.1111/j.1600-0625.2012.01560.x. [DOI] [PubMed] [Google Scholar]
- 12.Wurm EM, Lin LL, Ferguson B, et al. Exp Dermatol. 2012;21:676–81. doi: 10.1111/j.1600-0625.2012.01543.x. [DOI] [PubMed] [Google Scholar]
- 13.Bult CJ, Krupke DM, Eppig JT. Nucleic Acids Res. 1999;27:99–105. doi: 10.1093/nar/27.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krupke DM, Begley DA, Sundberg JP, et al. Nat Rev Cancer. 2008;8:459–65. doi: 10.1038/nrc2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begley DA, Krupke DM, Neuhauser SB, et al. Vet Pathol. 2012;49:218–23. doi: 10.1177/0300985810395726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das K, Tan P. Clin Genet. 2013;84:315–325. doi: 10.1111/cge.12229. [DOI] [PubMed] [Google Scholar]
- 17.Hunter KW, Crawford NPS. Annual Review of Genetics. 2008;42:131–141. doi: 10.1146/annurev.genet.42.110807.091659. [DOI] [PubMed] [Google Scholar]
- 18.Ron E, Michael D, Alex EL. Nucleic Acids Res. 2002;30:207–210. [Google Scholar]
- 19.Rustici G, Kolesnikov N, Brandizi M, et al. Nucleic Acids Res. 2013;41:D987–90. doi: 10.1093/nar/gks1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siolas D, Hannon GJ. Cancer Res. 2013;73:5315–5319. doi: 10.1158/0008-5472.CAN-13-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dadras SS, Silva KA, King LE, Jr, et al. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.47. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schofield PN, Gruenberger M, Sundberg JP. Vet Pathol. 2010;47:1016–20. doi: 10.1177/0300985810374845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schofield PN, Bard JB, Booth C, et al. Nucleic Acids Res. 2004;32:D512–5. doi: 10.1093/nar/gkh124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. “Pathology Image Record” of a tail-skin, transplantable melanoma from a LT.B6 mouse. This “Pathology Record”, in addition to displaying the histopathology image and associated clinical data, also shows the tumor, reference, and strain details.
Figure S2. Example of “Histology” and associated data for a skin melanoma PDX model.