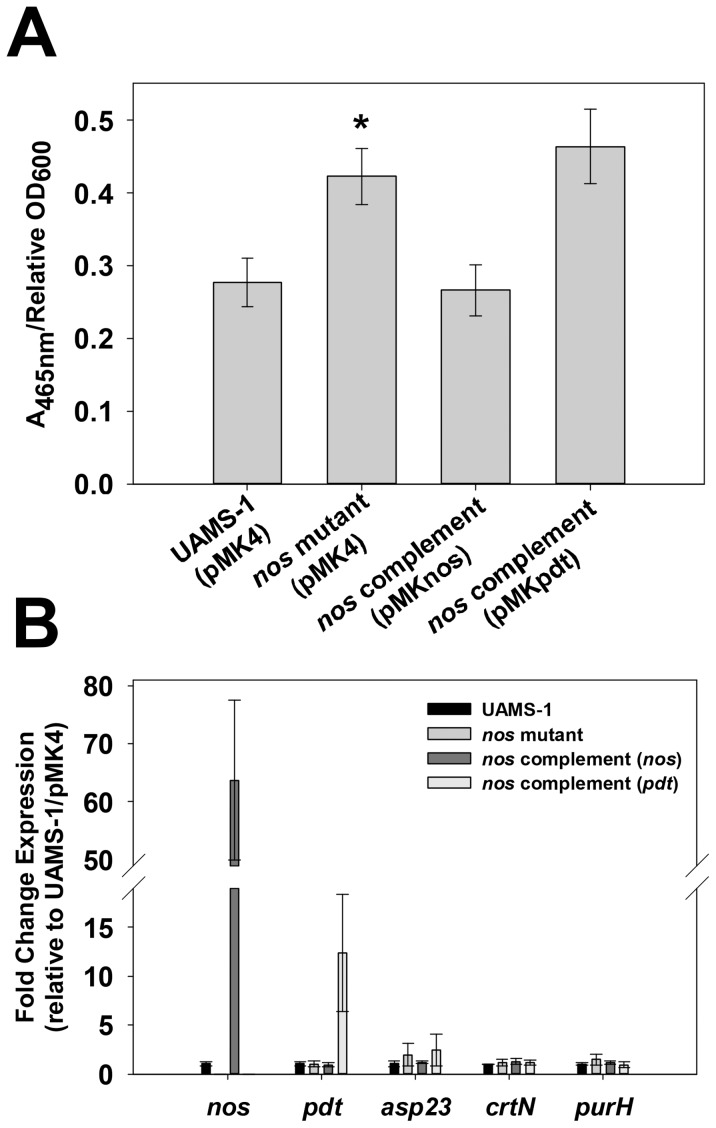
Figure 6. Effect of S. aureus nos mutation on carotenoid pigmentation.
A: UAMS-1 (pMK4; wild-type), KR1010 (pMK4; nos mutant), KR1010 (pMKnos; nos complement), and KR1010 (pMKpdt; nos mutant containing pdt complement plasmid) were grown for 48 hours at 37°C on TSA plates. Pigments from each culture were extracted with methanol, the A465 nm of each sample was measured, and standardized by dividing by the sample's corresponding relative OD600 value (measured prior to methanol extraction step). Results represent the average of n = 3 independent experiments, error bars = SEM. *statistical significance compared to UAMS-1 (pMK4) (p<0.05, Student-Newman-Keuls Test). B: Total RNA from 28 hour TSA plate cultures of UAMS-1 (pMK4), nos::erm mutant (pMK4), nos complement and pdt complement strains (n = 3 biological replicates each) was reversed-transcribed to cDNA, and subjected to quantitative real-time PCR using primers specific for detecting nos, pdt, asp23, crtN, and purH expression. The Livak (2-ΔΔCt) method was used to determine relative fold change expression of each gene, using measured sigA expression as the reference gene and the UAMS-1 (pMK4) sample as the calibrator.