Table 4. Asymmetric Synthesis of Aspartic Acid Derivativesa–d.
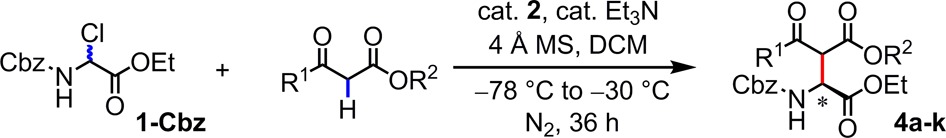
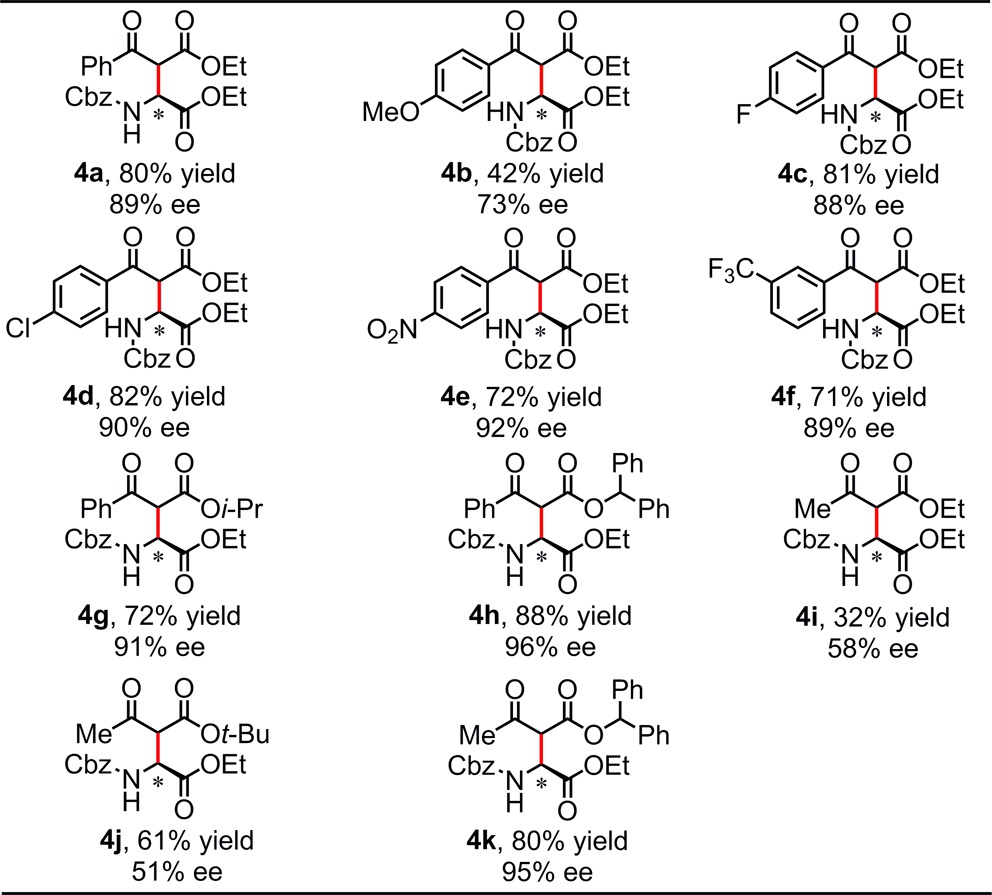
Conditions: substrate (0.25 mmol), catalyst (10 mol%), β-ketoester (0.5 mmol), 4 Å MS (40 mg), Et3N (25 mol%), DCM (5 mL), under N2, initially cooled to −78 °C and stirred at −30 °C, 36 h.
Isolated yield.
Products were isolated as the thermodynamic mixtures of diastereomers.
The structure and absolute configuration of 4h was established by X-ray crystallography, and the stereochemistry of all other products was assigned by analogy.
