Table 2. Cyclic Sulfamide Formation with Selected Alkenes.
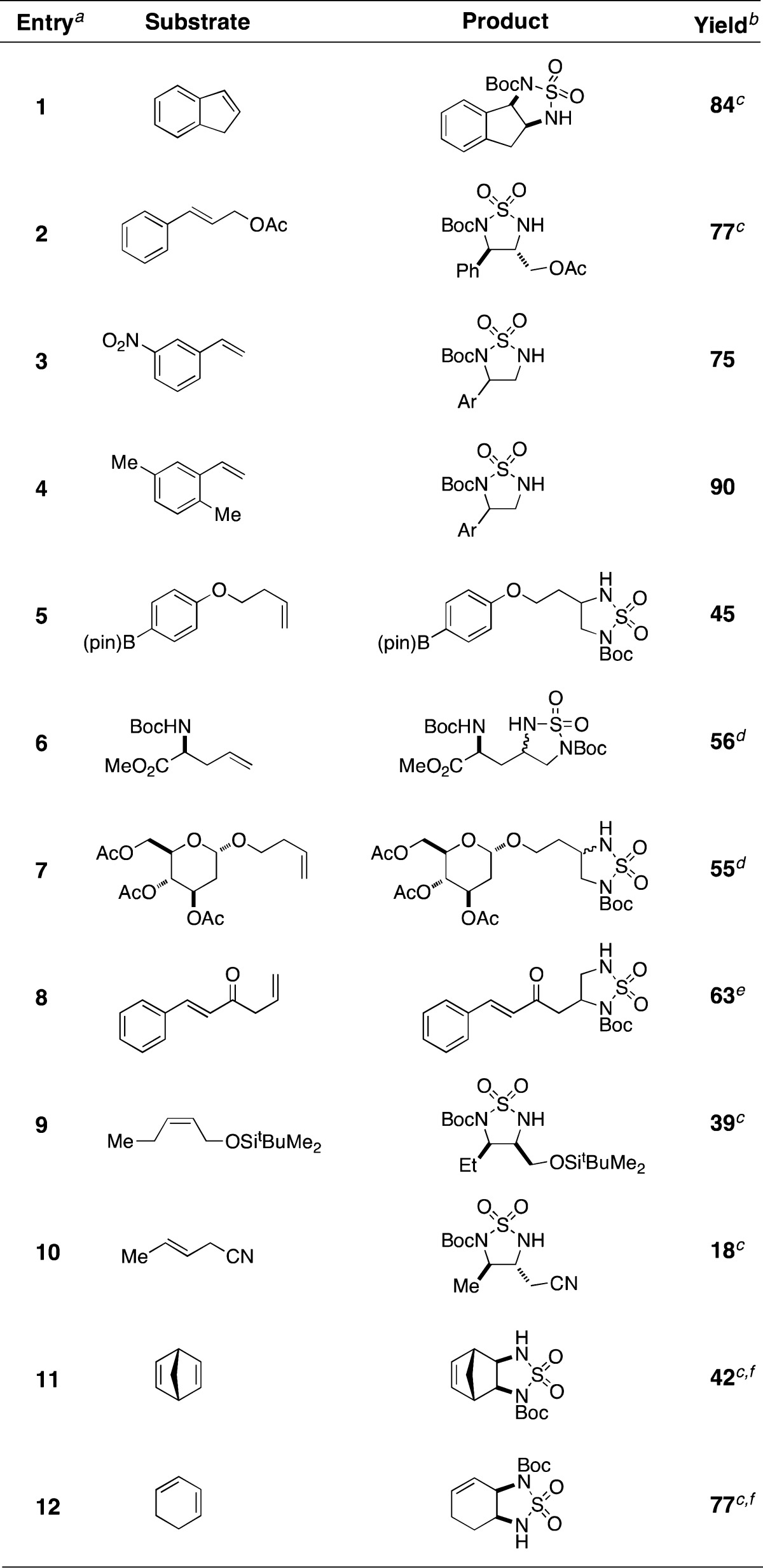
Reactions were performed in i-PrOAc with 1 equiv of substrate, 2 mol % Rh2(esp)2, 1.1 equiv of BocNHSO2NH2, 1.1 equiv of PhI(OAc)2, and 2.3 equiv of MgO. After the completion of the aziridination reaction, 1.1 equiv of NaI and DMF were added to induce rearrangement.
Isolated yields following chromatographic purification.
Product isolated as a single diastereomer.
dr = 1:1.
See ref (14).
Reaction performed with 10 equiv of substrate.
