Table 1. Copper-Catalyzed Migratory Cycloisomerization Reaction toward Borylated Furans.
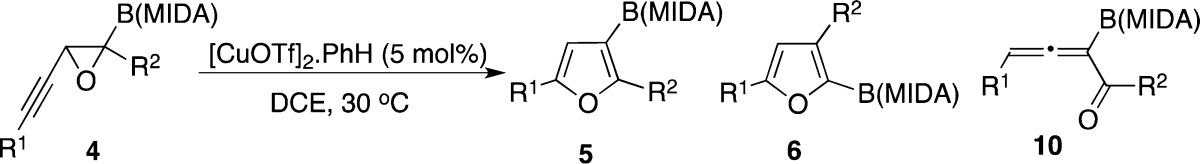
| entry | substrate | R1, R2 | yield, %a (5:6:10)b |
|---|---|---|---|
| 1 | 4a | nBu, H | 80 (97:3:0) |
| 2 | 4b | Ph, H | 36 (89:11:0)c |
| 3 | 4c | 2-OMe-Ph, H | 51 (99:1:0) |
| 4 | 4d | 2-CF3-Ph, H | 96 (0:0:100)d |
| 5 | 4e | nBu, nBu | 0e |
Isolated yields.
NMR ratios.
T = 55 °C.
Heating of the reaction did not lead to furan.
Decomposition of 4.
