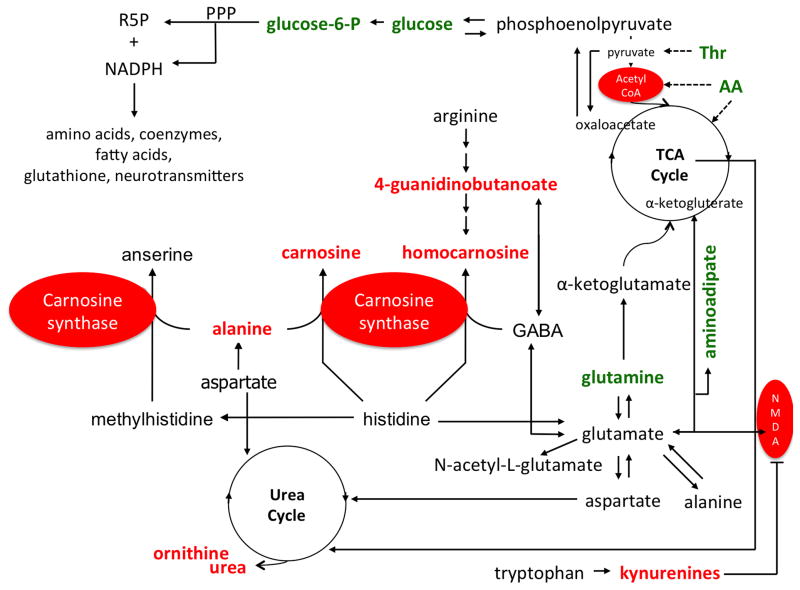
Figure 5. Metabolic pathways targeted by dietary LC-O3PUFA in the sham rat spinal cord.
Dietary LC-O3PUFAs target the metabolism of carnosine and homocarnosine, as evidenced by increased levels of alanine, carnosine, homocarnosine, and 4-guanidinobutanoate in the spinal cord of animals fed with LC-O3PUFAs. The diet rich in LC-O3PUFAs also altered the glutamine-glutamate cycling. A distinctive group of amino acid systems were affected by the diet, including threonine and tryptophan. In particular, animals fed with LC-O3PUFAs showed dramatic increases in the levels of kynurenines, which can regulate mitochondrial homeostasis and oxidative stress, inflammation, and glutamate excitotoxicity through NMDA receptor inhibition. Notably, the diet increased the levels of ornithine and urea, while decreasing glucose and glucose-6-P levels, showing selective alterations in the spinal cord cell bioenergetics. This support that LC-O3PUFAs fuel energy production largely by oxidative phosphorylation via the tricarboxylic acid (TCA) cycle and pentose phosphate pathway (PPP) rather than glycolysis, which are essential pathways for the synthesis of necessary macromolecules (i.e. amino acids, neurotransmitters, glutathione, nucleosides and lipids required for assembling new cells). These pathways may represent important mechanisms by which dietary LC-O3PUFAs confer prophylaxis against neurodegeneration and dysfunction in SCI. This reservoir of protective molecules and antioxidant bioavailability is expected to make neurons and glia more resistant against calcium overload, glutamate toxicity, and cell death following SCI. Metabolites in red increased with the dietary intervention. Features in green decreased with the LC-O3PUFA diet when compared to controls. Putative enzymatic/receptor targets are highlighted in red ovals. Abbreviations: AA, amino acid (polar); GABA, gamma-aminobutyrate; NADPH, nicotinamide adenine dinucleotide phosphate; NMDA, N-methyl-D-aspartate; PPP, pentose phosphate pathway; R5P, ribose 5-phosphate; Thr, threonine.