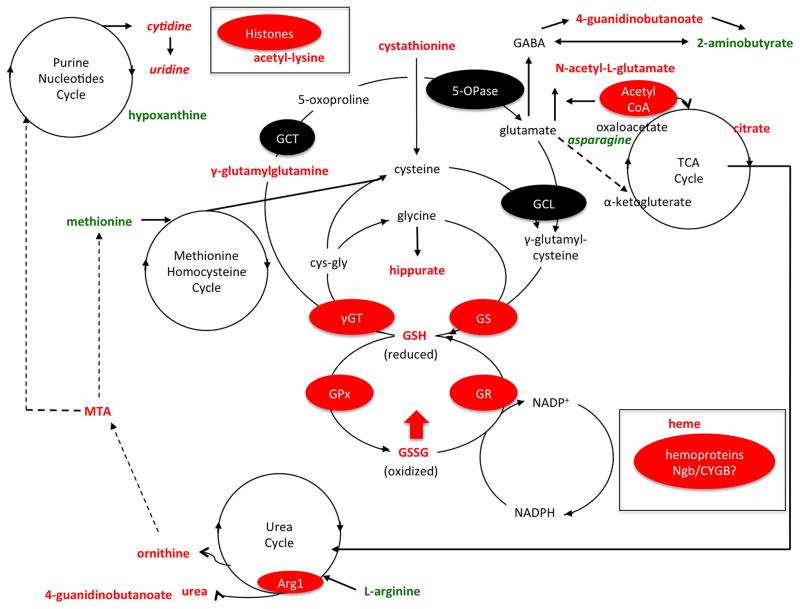
Figure 6. Metabolic pathways targeted by dietary LC-O3PUFA in the injured spinal cord.
Although we did not characterize the specific sources of ROS in the present study, our metabolomics dataset supports the role of mitochondrial dysfunction as a major source during both acute and chronic injury stages. Interestingly, we identified glutathione (GSH) metabolism as a molecular target of dietary LC-O3PUFAs. For instance, the animals receiving the dietary intervention showed increased spinal cord levels of γ-glutamylglutamine, cystathione, hippurate, GSH, and GSSH, suggesting increased production and/or reduced depletion of antioxidant pools after SCI. Notably, the levels of heme were increased in the spinal cord of rats exposed to the LC-O3PUFA-rich diet, proposing a novel protective mechanism for LC-O3PUFAs. Similar to the findings observed in sham rats, LC-O3PUFAs altered the TCA and Urea cycle. The increased levels of purine nucleotides and acetyl-lysine suggests a mechanism for which chronic dietary supplementation with LC-O3PUFAs modulates plasticity, growth, and gene expression. Metabolites in red increased with the dietary intervention. Features in green decreased with the LC-O3PUFA diet when compared to controls. Putative enzymatic and protein targets are highlighted in red ovals. Abbreviations: 5-OPase, 5-oxoprolinase; Arg1, arginase; CYGB, cytoglobin; cys-gly, cysteine-glycine; GSH, glutathione, reduced; GSSH, glutathione disulfide, oxidized; GCL, γ-glutamylcysteine ligase; GS, glutathione synthase; GPx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; γGT, γ-glutamyltransferase; GCT, γ-glutamylcylotransferase; MTA, 5′-methylthioadenosine; Ngb, neuroglobin; TCA, tricarboxylic acid.