FIGURE 2.
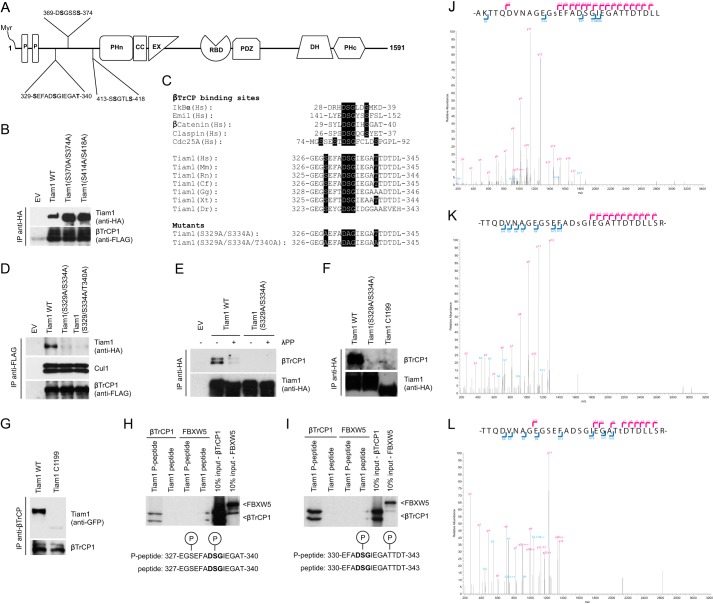
Phosphorylation of Ser329, Ser334, and Thr340 in Tiam1 is required for its interaction with βTrCP1. A, schematic representation of Tiam1 functional domains and three putative βTrCP-binding motifs. B, HEK293T cells were transfected with FLAG-tagged βTrCP1 and either HA-tagged wild type Tiam1, HA-tagged Tiam1(S370A/S374A), HA-tagged Tiam1(S414A/S418A), or an empty vector (EV). Forty-eight hours after transfection, cells were harvested and lysed. Whole cell extracts were subjected to immunoprecipitation (IP) with anti-HA resin, followed by immunoblotting with antibodies specific for the indicated proteins. C, alignment of the amino acid regions corresponding to the βTrCP-binding motifs in Tiam1 orthologs and previously reported βTrCP substrates, i.e. IκBα, Emi1, β-catenin, Claspin, and Cdc25A. The amino acidic sequences of the phosphodegron mutants are shown below. D, HEK293T cells were transfected with FLAG-tagged βTrCP1 and either HA-tagged wild type Tiam1, HA-tagged Tiam1(S329A/S334A), HA-tagged Tiam1(S329A/S334A/T340A), or an empty vector. Forty-eight hours after transfection, the cells were harvested and lysed. Whole cell extracts were subjected to immunoprecipitation with anti-FLAG resin, followed by immunoblotting with antibodies specific for the indicated proteins. E, HEK293T cells were transfected with an empty vector, HA-tagged wild type Tiam1, or HA-tagged Tiam1(S329A/S334A). Forty-eight hours after transfection, cells were harvested and lysed. Whole cell extracts were subjected to immunoprecipitation with anti-HA resin and then immunoblotted with antibodies specific for the indicated proteins. When indicated, immunocomplexes were incubated with lambda phosphatase (λPP) for 30 min and then washed. F, HEK293T cells were transfected with HA-tagged wild type Tiam1, HA-tagged Tiam1(S329A/S334A), or HA-tagged Tiam1-C1199. Whole cell extracts were subjected to immunoprecipitation and immunoblotting as in E. G, HEK293T cells were transfected with GFP-tagged wild type Tiam1 or GFP-tagged Tiam1-C1199. Cells were collected and lysed. βTrCP was immunoprecipitated from whole cell extracts, and immunocomplexes were analyzed by immunoblotting with antibodies specific for the indicated proteins. H and I, 35S-βTrCP1 and 35S-FBXW5 were transcribed/translated in vitro and incubated with beads coupled to peptides spanning the Tiam1 degron. Beads were washed with Triton-X buffer, and bound proteins were eluted and subjected to electrophoresis and autoradiography. The last two lanes correspond to 10% of the in vitro translated protein inputs. Peptide sequence spanning the Tiam1 degron is shown on the left. J–L, FLAG-HA-tagged Tiam1 was immunopurified from HEK293T cells and analyzed by mass spectrometry. Ion fragmentation spectra are shown. The indicated Tiam1 tryptic peptides spanning the phosphodegron were found phosphorylated on Ser329 (J), Ser334 (K), or Thr341 (L). As shown, peptide sequences can be explained by their respective collision-induced dissociation MS/MS spectra including Ser(P)329, Ser(P)334, and Thr(P)341.