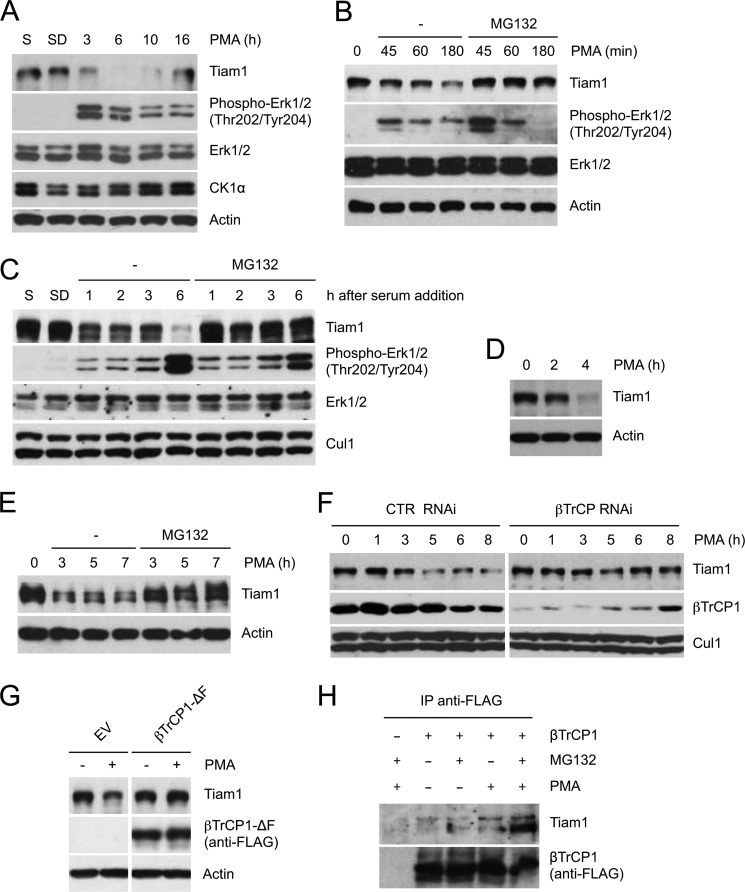
FIGURE 3.
Proteasome- and βTrCP-dependent degradation of Tiam1 in response to mitogens. A, HEK293 cells, cultured in low serum for 48 h, were treated with PMA. Cells were collected at the indicated times and lysed. Whole cell extracts were subjected to immunoblotting with antibodies specific for the indicated proteins. Actin is shown as a loading control. B, HEK293 cells were treated with PMA as in A with or without the proteasome inhibitor MG132. Whole cell extracts were analyzed as in A. C, HEK293 cells were either cultured in presence of serum (S) or deprived of serum for 48 h (SD). After serum readdition, the cells were collected at the indicated times. When indicated, the proteasome inhibitor MG132 was added. Protein extracts were analyzed by immunoblotting with antibodies specific for the indicated proteins. Cul1 is shown as a loading control. D and E, T98G cells (D) and MDCK cells (E) were treated with PMA as in A and with MG132 when indicated. Whole cell extracts were analyzed as in A. F, HEK293 cells were transfected with the indicated siRNA oligonucleotides and treated as in A. Cul1 is shown as loading control (CTR). G, HEK293 cells were transfected with FLAG-tagged βTrCP1-ΔF or an empty vector (EV) and treated with PMA for 2 h. Cells were collected and lysed. Whole cell extracts were analyzed as in A. H, HEK293 were transfected with an empty vector or FLAG-tagged βTrCP1. Forty-eight hours after transfection, cells were treated with MG132 and PMA for 5 h (when indicated), then harvested, and lysed. Whole cell extracts were immunoprecipitated (IP) with anti-FLAG resin and immunoblotted with anti-Tiam1 and anti-FLAG antibodies.