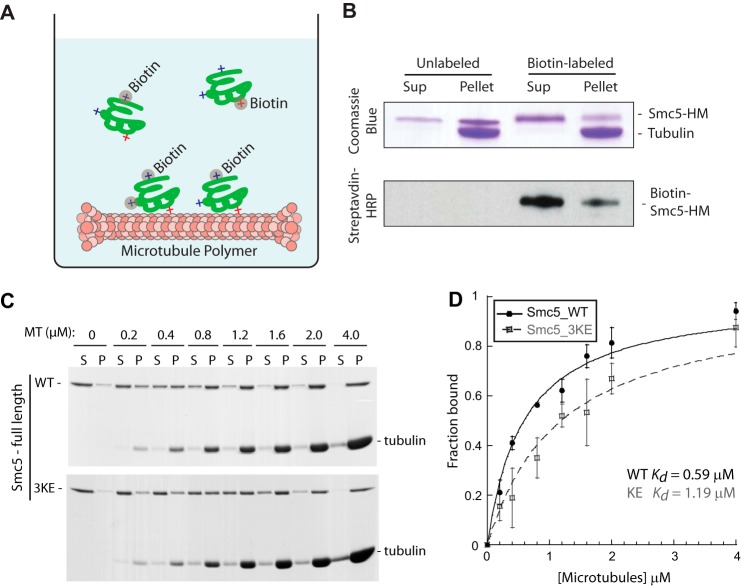
FIGURE 3.
Generation of a microtubule-binding mutant of SMC5. A, schematic depicting the method used to identify surface-exposed lysine residues on Smc5 that are essential (red +) or non-essential (blue +) for microtubule binding. Upon chemical labeling by biotin, modified lysines essential for binding will be enriched in the supernatant fraction. Relative abundances of tryptic peptides containing the modified lysines in the supernatant and pellet fractions were quantified by mass spectrometric analysis. B, Coomassie Blue-stained gel and streptavidin-HRP blot of the supernatant and pellet fractions of mock-labeled or biotin-labeled Smc5-HM proteins from the microtubule co-sedimentation assay. The enrichment of labeled Smc5-HM in the supernatant indicates the perturbation of microtubule binding upon lysine modifications. C, three lysine residues in the hinge region of Smc5 identified by the method illustrated above in A and B were mutated into glutamic acid to generate the microtubule-binding mutant called Smc5-3KE. Coomassie Blue-stained microtubule titration gels of full-length Smc5-WT (wild-type) or Smc5-3KE from the co-sedimentation assay are shown. D, fractions of microtubule-bound Smc5-WT (red) and Smc5-3KE from four independent microtubule co-sedimentation experiments were plotted against microtubule concentrations and fitted to a hyperbola to determine their dissociation constants (Kd).