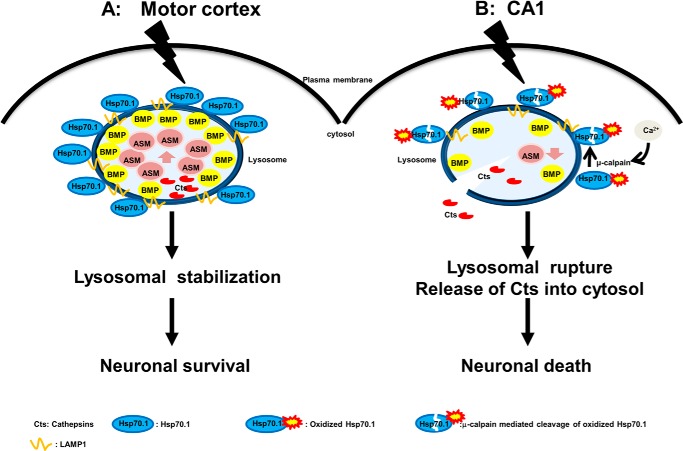
FIGURE 10.
The role of Hsp70.1 affecting neuronal fate by differentially regulating ASM. A, in the postischemic motor cortex, up-regulated Hsp70.1 is present at lysosomal membranes and then binds to BMP, where μ-calpain activation and decreased BMP levels did not occur. Binding Hsp70.1 to BMP enhances ASM activity in vivo and facilitates lysosomal stabilization, resulting in protection of the motor cortex from neuronal cells death. B, in the postischemic CA1, cerebral ischemia causes μ-calpain activation and a concomitant reduction in both the lysosomal membrane localization of Hsp70.1 and BMP levels. Furthermore, the lipid binding domain of Hsp70 may be disrupted by activated μ-calpain-mediated cleavage of the oxidized Hsp70. Since this may diminish Hsp70-BMP binding, the resultant decrease in ASM activity in vivo may increase lysosomal rupture with leakage of cathepsin B into the cytosol and neuronal death.