FIGURE 2.
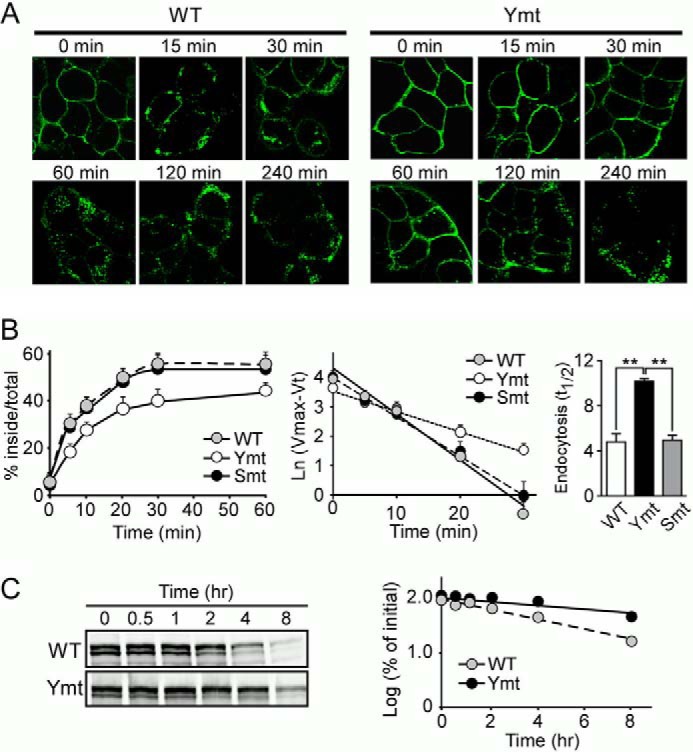
Mutation of tyrosine-based motifs in the LRP6 cytoplasmic tail impairs its endocytosis. A, HEK293 cells stably expressing the LRP6 WT or tyrosine mutant (Ymt) were incubated with Alexa Fluor 488-labeled anti-HA IgG for 1 h at 4 °C and then shifted to 37 °C for increasing periods of time to initiate endocytosis. Fluorescence images were obtained by confocal microscopy. Scale bars, 5 μm. B, cells stably expressing LRP6 WT, tyrosine (Ymt), and serine/threonine mutants (Smt) were subjected to a kinetic analysis of endocytosis using 125I-labeled Mesd (5 nm). The sum of ligand that was internalized, and that which remained on the cell surface at the end of the assay was used as the maximum potential internalization. The fraction of internalized ligand after each time point was calculated and plotted (left panel). Data represent mean ± S.D. Then, Ln (Vmax (maximum internalization) − Vt (internalization % at each time point)) was calculated and plotted against each time point (middle panel). The half-time (t½) for the internalization of LRP6 was then calculated as t½ = ln2/k = 0.693/(slope) and is shown as a bar graph (right panel). Data represent mean ± S.D. **, p < 0.01. C, cells stably expressing LRP6 WT or tyrosine mutant were treated with cycloheximide and lysed at 0, 0.5, 1, 2, 4, and 8 h following the treatment. Lysates were then immunoblotted for LRP6. The half-life of LRP6 was calculated on the basis of the slope of the semilog plot of percentage of change in LRP6 versus time.
