FIGURE 3.
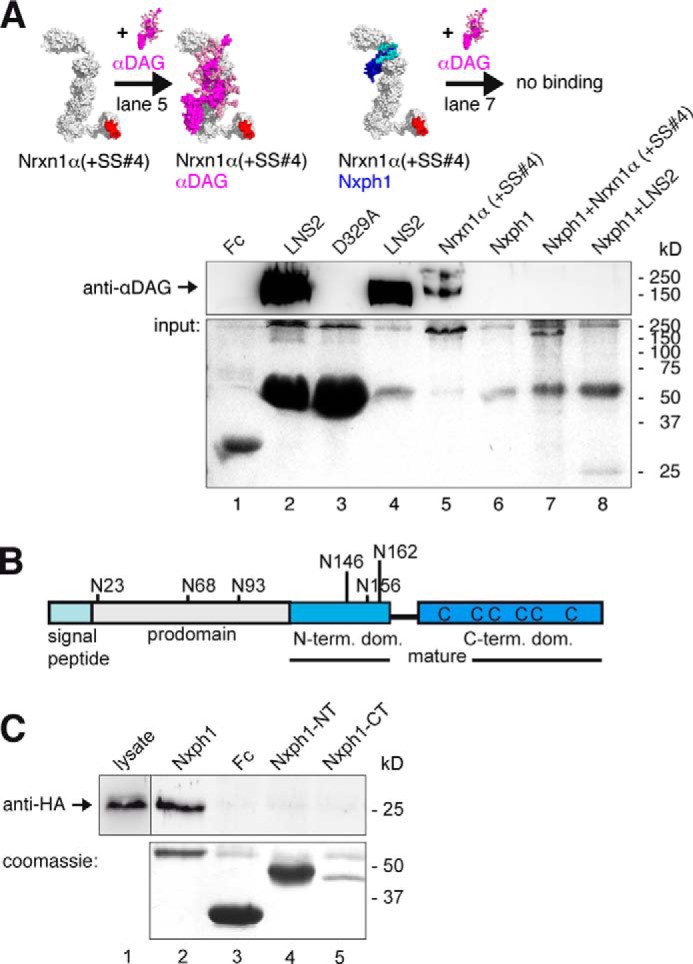
Nxph1 in complex with α-Nrxn prevents binding of αDAG to LNS2. A, pictograms of interactions probed (color-coded as labeled; splice inserts are in red) and representative immunoblot. Endogenous αDAG from N2a cells binds to Fc-tagged extracellular domain of Nrxn1α (lane 5) and to isolated Fc-LNS2 (lanes 2 and 4). Preformed complexes of Fc-tagged Nxph1·Nrxn1α (lane 7) or Nxph1·LNS2-HA (lane 8) prevent αDAG binding. Protein staining after pull-down (lower panel) shows that low amounts of LNS2-Fc (∼55 kDa) are sufficient to bind αDAG (lane 4), but LNS2-HA (25 kDa) in complex with Nxph1-Fc (∼55 kDa) does not (lane 8). Comparable amounts of Nrxn1α-Fc (∼220 kDa, lane 5) and Nrxn1α (∼165 kDa, lane 7) were used. Isolated Nxph1-Fc does not bind to αDAG (lane 6). LNS2 and glycosylated mature Nxph1 are both ∼25 kDa (lane 8 and Fig. 2B). B, preproprotein with signal peptide (cyan) and prodomain (gray) is cleaved to generate mature Nxph1, consisting of an N-glycosylated N-terminal (light blue) and a cysteine-rich C-terminal (dark blue) domain. C, binding of Fc-tagged Nxph1 to HA-tagged LNS2 by co-expression in COS7 cells (lane 1) requires complete mature protein (lane 2), as isolated N- (lane 4) or C-terminal domains (lane 5) are not sufficient. The Fc-tagged glycosylated N-terminal domain has a similar size as the non-glycosylated C-terminal domain. The size of molecules in A are to scale, and the modeled complexes αDAG·αNrxn and Nxph1·αNrxn were generated using two criteria, (i) coverage of hot spots and (ii) maximal surface area buried (see “Experimental Procedures”). Note that in addition to complexes shown, other conformations are not excluded.
