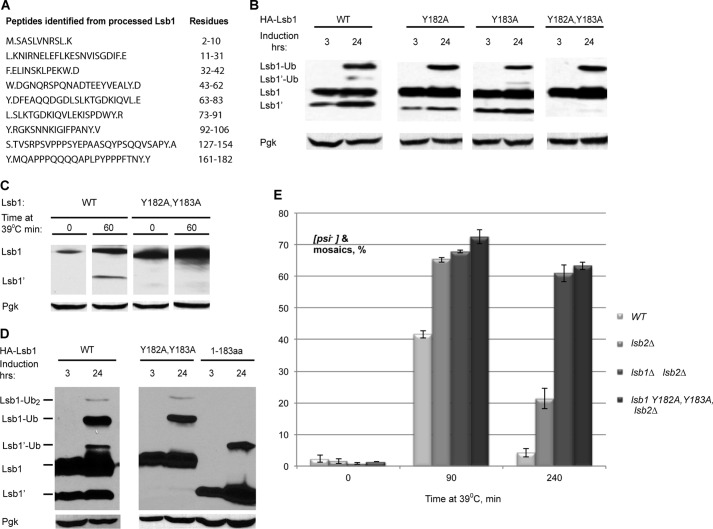
FIGURE 4.
Lsb1 is processed at residues Tyr-182,Tyr-183. A, detection of the C-terminal peptide of processed Lsb1 protein by LC-MS/MS. Y.MQAPPPQQQQAPLPYPPPFTNY.Y was identified as the potential C-terminal peptide. B, Y182A,Y183A substitution blocks Lsb1 processing. HA-tagged wild type, single, and double mutants of Lsb1 were induced from PCUP1 for the indicated period of time and analyzed by immunoblotting with anti-HA Ab. C, thermal stress induces processing of wild type but not the Y182A,Y183A mutant. Lsb1 proteins are expressed from the endogenous promoter. Protein levels were analyzed at the indicated time points using anti-Lsb Ab. D, molecular weights of the unprocessed and processed forms of Lsb1 are equal to molecular weights of the full size Y182A,Y183A mutant Lsb1 protein and truncated Lsb1 protein (1–183 amino acids), respectively, as judged from the SDS-PAGE gel. Wild type, mutant, and truncated proteins, tagged with HA at N termini, were expressed from the copper-inducible promoter for the indicated periods of time. Protein extracts were run on SDS-PAGE gel and detected with the anti-HA antibody. Pgk1 protein detected by anti-Pgk1 Ab was used as a loading control (B–D). E, double substitution Y182A,Y183A in LSB1 (in combination with lsb2Δ) affects [PSI+] destabilization and recovery during HS the same way as lsb1Δ. Yeast were grown and treated by HS as in Fig. 1B.