FIGURE 1.
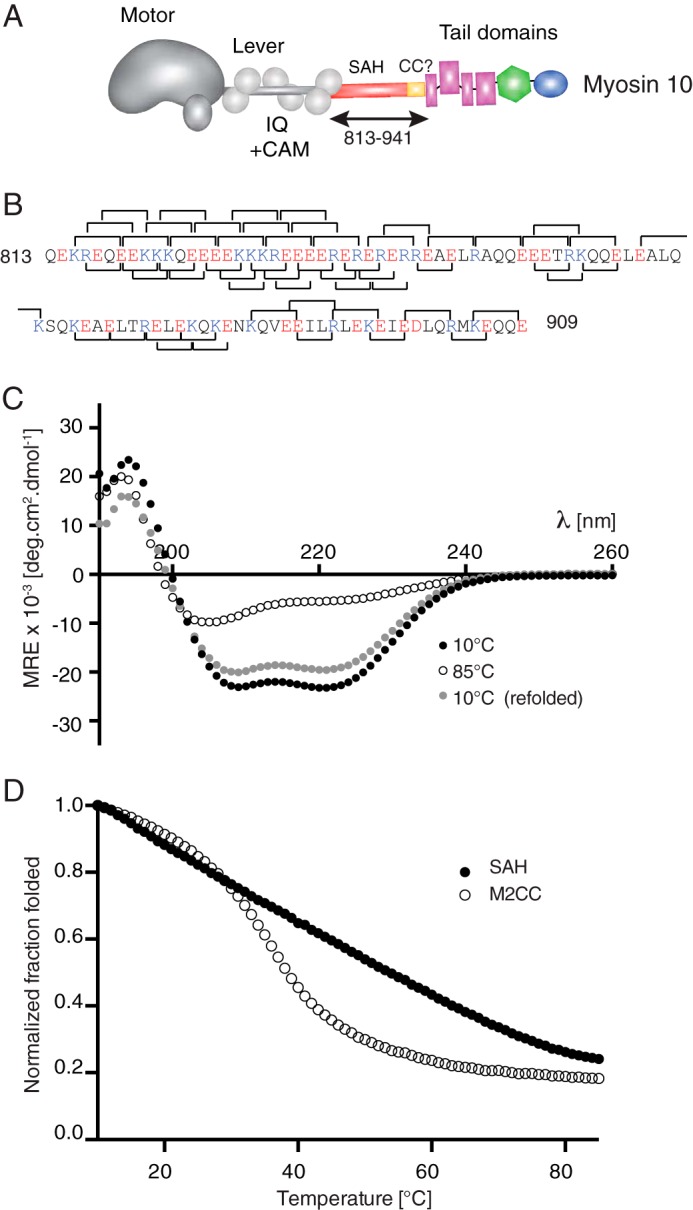
Intrahelical ionic interactions and circular dichroism of the predicted SAH domain from myosin-10. A, the domains found in myosin-10 showing the position of the predicted SAH domain and the antiparallel coiled coil (CC) (residues 813–941) between the lever and the tail domains. B, the sequence of the predicted SAH domain used in all the experiments here showing the potential i, i + 4 interactions and i, i + 3 interactions as brackets above and below the sequence, respectively. Basic residues (Lys and Arg) are shown in blue, and acidic residues (Glu and Asp) are shown in red. C, mean spectra for the SAH domain from myosin-10 at temperatures of 10 and 85 °C and then after returning to 10 °C. Those collected at 10 °C show profiles typical for α-helical proteins with >80% reversibility after thermal melting. Mean values from n = 6 repeats are shown. The helical content was calculated from the measured MRE values at 222 nm to be 67%. D, thermal melting curves for the SAH domain compared with that for a known coiled coil (M2CC). For each species, MRE222 values have been normalized to the value at 10 °C. CAM, calmodulin; deg, degrees.
