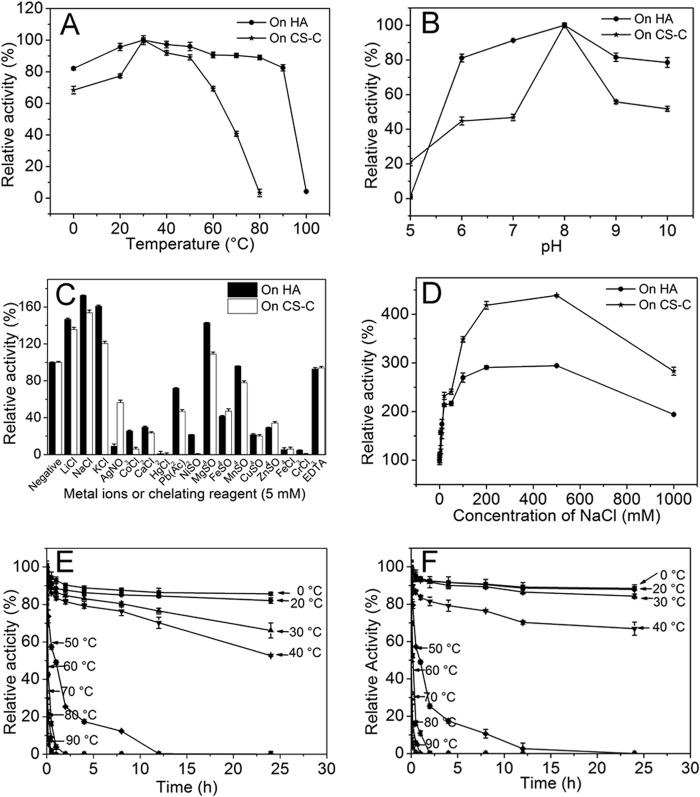
FIGURE 3.
Biochemical reaction conditions for recombinant rHCLase. A, effects of temperature. The enzyme activities of rHCLase were measured using HA and CS-C as substrates in the 50 mm NaH2PO4-Na2HPO4 buffer (pH 8.0) at different temperatures for 10 min. Data are shown as the percentage of the activity of that obtained at 30 °C (100%). B, effects of pH. The activities of rHCLase against HA and CS-C were measured in buffers with varying pH values from 5 to 10 at 30 °C for 10 min. Data are shown as the percentage of the activity of that obtained in the NaH2PO4-Na2HPO4 buffer (pH 8.0). C, effects of metal ions. The activities of rHCLase against HA and CS-C were measured in the NaH2PO4-Na2HPO4 buffer (pH 8.0) containing a 5 mm concentration of various metal ions at 30 °C for 10 min. Data are shown as the percentage of the activity of that obtained in the buffer without tested metal ions. D, effects of NaCl concentrations. The activities of rHCLase against HA and CS-C were measured in the NaH2PO4-Na2HPO4 buffer (pH 8.0) containing 0-1 m NaCl at 30 °C for 10 min. Data are shown as the percentage of the activity obtained in the buffer without NaCl. E and F, thermostability of rHCLase. The enzyme in 50 mm NaH2PO4-Na2HPO4 buffer (pH 8.0) was preincubated for 0–24 h at temperature from 0 to 90 °C, and the residual activity against HA (E) or CS-C (F) was estimated at 30 °C. Data are shown as the activity relative to that of untreated rHCLase. Error bars represent means of triplicates ±S.D.