FIGURE 3.
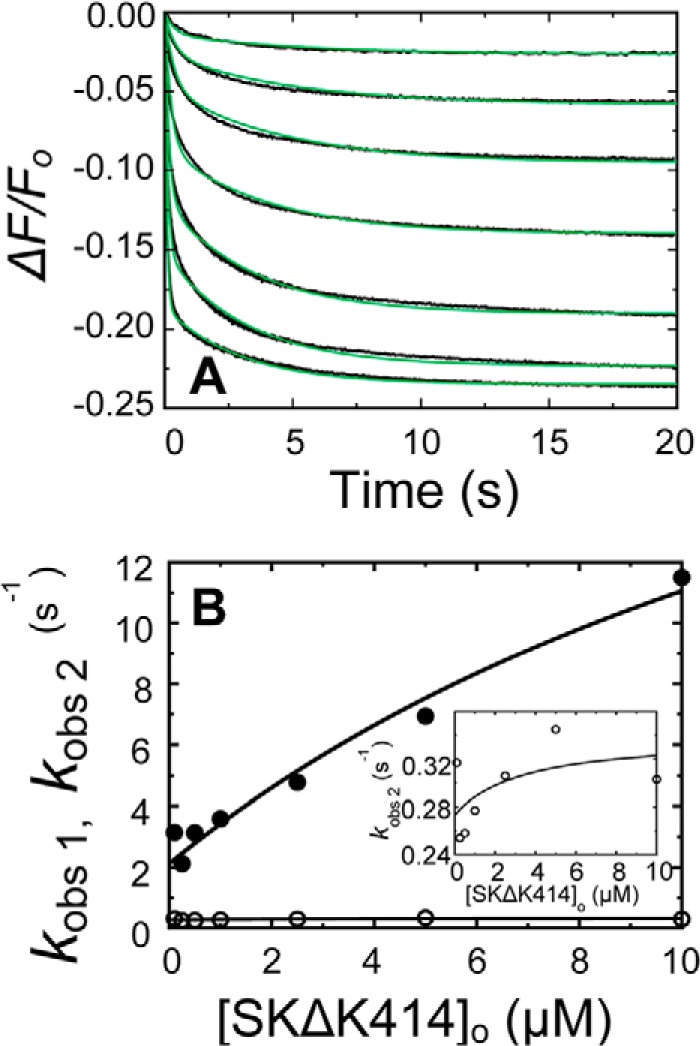
Kinetics of SKΔK414 binding to [5F]FFR-[Lys]Pg in the absence of lysine analogs. A, the fractional fluorescence intensity changes (ΔF/Fo) following rapid mixing of [5F]FFR-[Lys]Pg and SKΔK414 versus time are shown in the absence of lysine analogs at 20 nm [5F]FFR-[Lys]Pg and 0.1, 0.25, 0.5, 1.0, 2.5, 5.0, and 10 μm SKΔK414. Green solid lines represent the fits from numerical integration as described under “Experimental Procedures.” B, dependences of kobs 1 and kobs 2 (● and ○) on the total SKΔK414 concentration ([SKΔK414]o) are shown for binding to 20 nm [5F]FFR-[Lys]Pg. The inset shows the kobs 2 dependence on an enlarged scale. Solid lines represent the fits by Equation 2 with the parameters given in Table 1. Experiments were performed and analyzed as described under “Experimental Procedures.”
