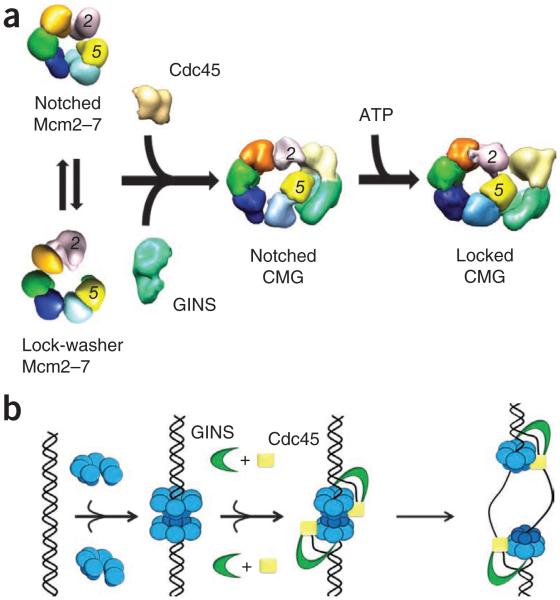
Figure 8.
Model for Mcm2–7 activation and function. (a) Free Mcm2–7 can exist in either an open, lock-washer or notched, planar configuration. Each form shows a discontinuity between Mcm2 and Mcm5. Binding of GINS–Cdc45 stabilizes the notched, planar Mcm2–7 state, whereas ATP binding promotes ring closure. (b) ORC, Cdc6 and Cdt1 load a preopened Mcm2–7 assembly onto dsDNA as an inactive double hexamer. GINS and Cdc45 bind to Mcm2–7, concomitant with an isomerization that creates or stabilizes melted DNA. The side channel formed by the GINS–Cdc45 subcomplex likely prevents DNA escape from Mcm2–7 and may help partition the lagging DNA strand from its complement following unwinding.