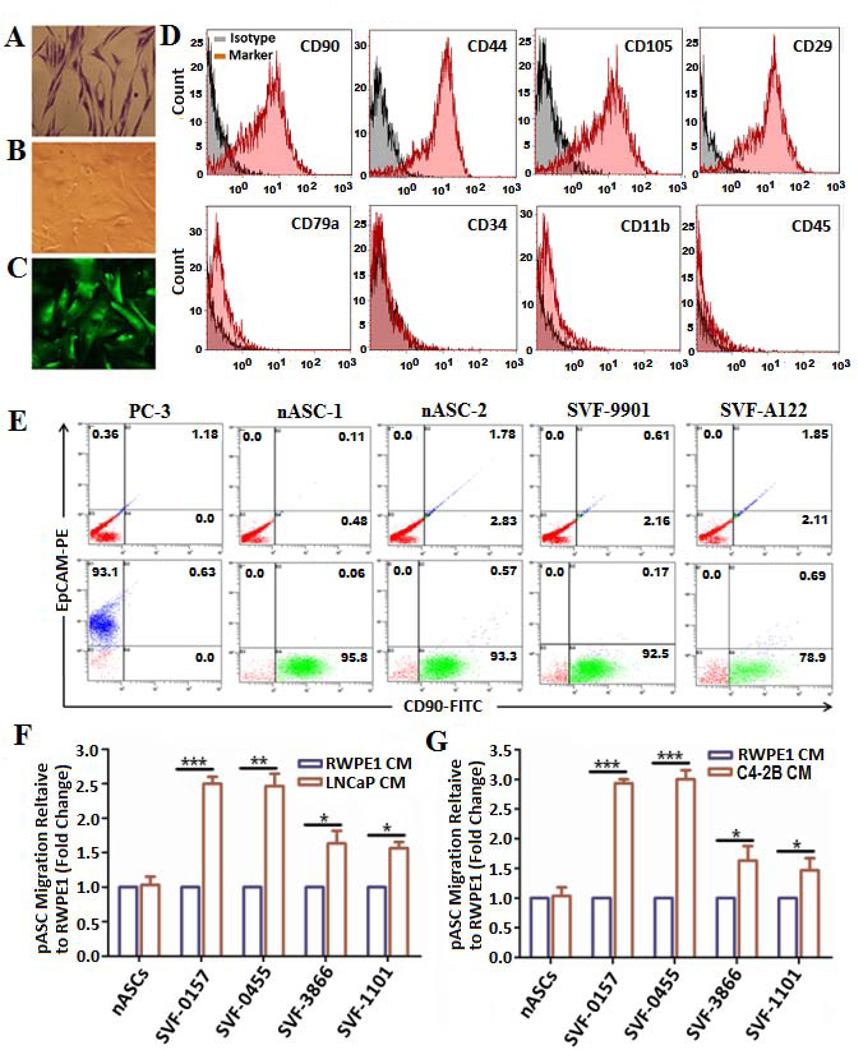
Figure 1. Isolation, characterization and transendothelial migration of patient-derived ASCs towards PC cell-CM in vitro.
Representative photomicrographs of methylene blue stained patient-derived ASCs (pASCs) depicting retention of fibroblast-like phenotype (A) in comparison to parental cells stably transduced with a lentivirus construct expressing green fluorescent protein (pLV-eGFP) under bright field (B) and fluorescence microscope (C) (40x). (D) Purity of isolated pASCs was verified by FACS analysis of mesenchymal surface expression markers CD90, CD44, CD105, and CD29 (upper panel) and of hematopoietic lineage markers CD79α, CD34, CD11b, and CD45 (lower panel) compared with their isotype controls. (E) Representative FACS analysis of CD90-FITC and the epithelial marker EpCAM-PE to determine purity of pASCs (designated SVF-9901 and SVF-A122) against normal ASCs (nASC-1 and nASC-2) and PC-3 cells (lower panel) versus control isotype (upper panel). (F, G) Transendothelial migration of pLV-eGFP-labeled nASCs and pASCs (SVF-0157, SVF-0455, SVF-3866, and SVF-1101) through a confluent layer of hBMEC-1/basement membrane barrier towards CM of LNCaP, C4-2B, or RWPE-1 cells in the lower chambers. The pASCs migration was monitored in quadruplicates after 24 h by a fluorescence plate reader, normalized to RWPE-1 cells and expressed as a fold change in fluorescence intensity relative to nASCs by three independent experiments. * p<0.05, **p<0.01 and *** p<0.001.