Abstract
Background Many clients of HIV care and treatment services have unmet contraceptive needs. Integrating family planning (FP) services into HIV services is an increasingly utilized strategy for meeting those unmet needs. However, numerous models for services integration are potentially applicable for clients with diverse health needs. This study developed and tested a ‘facilitated referral’ model for integrating FP into HIV care and treatment in Tanzania with the primary outcome being a reduction in unmet need for contraception among female clients.
Methods The facilitated referral model included seven distinct steps for service providers. A quasi-experimental, pre- and post-test, repeated cross-sectional study was conducted to evaluate the impact of the model. Female clients at 12 HIV care and treatment clinics (CTCs) were interviewed pre- and post-intervention and CTC providers were interviewed post-intervention.
Results A total of 323 CTC clients were interviewed pre-intervention and 299 were interviewed post-intervention. Among all clients, the adjusted decrease in proportion with unmet need (3%) was not significant (P = 0.103) but among only sexually active clients, the adjusted decrease (8%) approached significance (P = 0.052). Furthermore, the proportion of sexually active clients using a contraceptive method post-intervention increased by an estimated 12% (P = 0.013). Dual method use increased by 16% (P = 0.004). Increases were observed for all seven steps of the model from pre- to post-intervention. All providers (n = 45) stated that FP integration was a good addition although there were implementation challenges.
Conclusion This study demonstrated that the facilitated referral model is a feasible strategy for integrating FP into HIV care and treatment services. The findings show that this model resulted in increased contraceptive use among HIV-positive female clients. By highlighting the distinct steps necessary for facilitated referrals, this study can help inform both programmes and future research efforts in services integration.
Keywords: HIV, family planning, service integration, referrals, Tanzania
KEY MESSAGES.
Integrating family planning (FP) services into HIV care and treatment services is an increasingly utilized strategy for meeting the contraceptive needs of HIV-positive women and couples.
Numerous models for services integration are potentially applicable for clients with diverse health needs depending on the health service context.
A seven-step facilitated referral model of integration that was developed, implemented and tested for this FP–HIV integration study resulted in a significant increase in contraceptive use among HIV-positive female clients.
The facilitated referral model is a feasible strategy for FP–HIV services integration with broader implications for the integration of other health services.
Introduction
People living with HIV have health needs beyond those directly associated with their HIV infection. Mounting evidence shows that many people with HIV have unmet contraceptive needs and unintended pregnancies. For example, 53% and 62% of pregnancies among HIV-positive women were unintended in recent studies in Uganda and South Africa, respectively (Wanyenze et al. 2011; Schwartz et al. 2012), and up to 35% of female clients on antiretroviral therapy (ART) in Nigeria had unmet contraceptive needs (McCarraher et al. 2011). Meanwhile, some people with HIV want more children but need information about how to increase the likelihood of safe conception and to reduce the chances of mother-to-child transmission of HIV when they do get pregnant. Studies in Uganda and South Africa showed that 11% and 26% of HIV-positive women desired more children, respectively (Kipp et al. 2011; Myer et al. 2007).
Providing family planning (FP) services in HIV care and treatment clinics (CTCs) offers an opportunity to increase access to contraception among women and couples living with HIV. FP is also a cost-effective strategy for preventing vertical HIV transmission (Sweat et al. 2004; Reynolds et al. 2006; Halperin et al. 2009). In the past decade, international organizations have endorsed the integration of FP and HIV services as a strategy to address unmet contraceptive need among women living with HIV and as one prong of prevention of mother-to-child transmission initiatives (PMTCT) (Wilcher and Cates 2010; WHO 2010; UNAIDS 2010; United Nations General Assembly 2011). A small but growing body of evidence suggests that integrated services can lead to improvements in access to and quality of care, programme efficiency, provider knowledge and skills, reductions in stigma, and an increase in contraceptive use (GNP+ et al. 2008; Spaulding et al. 2009; Church and Mayhew 2009; Kennedy et al. 2010). However, the potential public health benefits of integrated services remain largely undocumented. Integrated service models are challenging to implement and few evaluations of scalable, replicable programmes have been conducted (Spaulding et al. 2009; Sweeney et al. 2012). Integration efforts often face unclear and unenforced guidelines and policies, a lack of leadership, and inadequately trained supervisors and providers who often have heavy workloads with few incentives (Mayhew et al. 2000; Oliff et al. 2003; PATH 2007; Church and Mayhew 2009; Dickinson et al. 2009; Adamchak et al. 2010; Smit et al. 2012). In addition, service integration is challenged by monitoring systems that have yet to be integrated, weak referral systems, inadequate infrastructure, commodity stock-outs and poor procurement systems, and vertical financing structures (Mayhew et al. 2000; Oliff et al. 2003; French et al. 2006; PATH 2007; Church and Mayhew 2009; Chabikuli et al. 2009; Dickinson et al. 2009; Chibwesha et al. 2011).
Service integration is not unique to HIV and FP services. Debates regarding vertical vs horizontal organization of services and the implications for serving the varied healthcare needs of patients have been long ongoing in the field of global public health and the service delivery issues are often similar across health conditions (Mills 1983; Atun et al. 2010a,b; Shigayeva et al. 2010). Using HIV care and treatment services and FP services as an example, multiple approaches for organizing integrated service delivery could exist along a continuum—from a single HIV service provider offering FP counselling and contraception to a client, to a HIV care and treatment client receiving FP services alongside her HIV services but from multiple providers, to referral-based approaches, where HIV service providers encourage clients to seek FP counselling and methods at a separate clinic with separate providers (Shigayeva et al. 2010; Atun et al. 2010b; Kuhlmann et al. 2010). The appropriateness and feasibility of a particular integration approach is contingent upon factors such as available human resources, facility infrastructure, provider capacity, the commodity supply chain and the nature of the HIV epidemic in an area (Church and Mayhew 2009; Kennedy et al. 2010; Atun et al. 2010a,b). To meet the FP needs of HIV-positive clients attending HIV care and treatment services, health systems and programmes must have the evidence to guide them on the appropriate service integration model feasible for their local context.
In settings where it is not feasible for HIV CTCs to provide ‘one-stop-shops’ that include comprehensive FP services for clients, referral-based approaches can capitalize on existing ‘vertical’ services offered within the primary health system. ‘Facilitated referrals’, or accompanied referrals, are enhanced referrals that include components that may support completion of a referral by strengthening the linkages between two services or between community-based and facility-based services. Facilitated referrals are distinguished from typical referrals by encouraging compliance with the referral with same-day support, recording and monitoring of referral follow-through, and addressing barriers to the referral such as financial or transportation support, or accompanying the client on the referral (Winch et al. 2005). While this approach is not a new idea, few rigorous evaluations of this service integration model in different health settings have been completed and the existing evidence of the effectiveness of this facilitated referral approach for HIV–FP integration is mixed.
As part of a cluster-randomized trial in Zambia (Gill et al. 2011), traditional birth attendants (TBAs) used a combination of antibiotic administration and facilitated referrals to health centres for infants with possible sepsis. The intervention arm of the study experienced more than twice as many referrals for infants compared with the control group; however, infant mortality due to infections was similar between the two groups. Study authors highlighted that the intervention served to link clients of TBAs with facility-based health services. Another study in Mozambique (Ciampa et al. 2011) examined the impact of enhanced referrals on early infant HIV diagnosis. HIV-positive postpartum women were accompanied to private counselling on infant HIV screening before discharge from the maternity ward, and a medical record for the infant was generated. The facilitated referral intervention significantly increased the odds of mothers returning with their infants for screening and reduced the median time to screening.
Facilitated referrals were also used for women receiving services for sexually transmitted infections (STIs) in the USA (Shlay et al. 2003) and for women receiving HIV services in Nigeria (McCarraher et al. 2011) to increase their access to contraception. In the USA, STI clinic clients were randomized to either individual FP counselling and facilitated referrals, which included help selecting a physician, scheduling the appointment and follow up on completion of the appointment, or to the standard of care, which included basic information on contraception and a list of FP providers. Among women receiving enhanced services, both FP use and dual method use significantly increased. In Nigeria, HIV care and treatment clients were randomized to either enhanced FP services with same-day escorted referrals or a standard referral to the co-located FP services. Among HIV-positive women receiving enhanced services, modern method use increased, but no difference was found in contraceptive use between women receiving enhanced services (11.0%) and women who received a standard referral (11.6%).
While the evidence for facilitated referrals has shown some positive impact on improving health care access and service utilization, the evidence is still limited. In particular, a significant gap remains in the literature regarding the necessary steps of an effective facilitated referral process. Specification of these steps is important in order to adequately test a model of facilitated referrals for HIV–FP service integration as well as to inform future integrated service models for replication or scale-up if the model is found effective.
Tanzania
The United Republic of Tanzania was a highly appropriate setting in which to develop and test a model of facilitated referrals for HIV and FP services integration. The national prevalence of HIV among 15- to 49-year-olds is 5.7%, the total fertility rate is 5.4, and the unmet need for contraception among all women is 18.3% and 25.3% for currently married women (TACAIDS et al. 2008; National Bureau of Statistics [Tanzania] and ICF Macro 2011). From 2006 to 2011, FHI 360 was the HIV/AIDS care and treatment technical lead for the TUNAJALI programme, supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) and the United States Agency for International Development (USAID) and led by Deloitte. TUNAJALI, meaning ‘We Care’, provided technical assistance to the government to strengthen home-based care and orphans and vulnerable children support services along with support in rolling out national care and treatment programmes (Deloitte 2010). TUNAJALI supported 33 CTCs in four regions and by March 2011 the programme had enrolled nearly 73,000 patients with over 48,000 using antiretroviral drugs (Deloitte 2011).
In 2006, an assessment of three TUNAJALI-supported CTCs found that over half of sexually active ART clients had an unmet need for contraception and almost a third desired pregnancy (Mpangile et al. 2006). The Government of Tanzania through the National AIDS Control Program (NACP) responded to this assessment with strong support for the integration of comprehensive FP services into their care and treatment training curricula. Additionally, the NACP, in conjunction with the Reproductive and Child Health Section (RCHS) of the Ministry of Health and Social Welfare (MOHSW), requested FHI 360 to develop and test a facilitated referral model for integrating FP and HIV care and treatment services. Although various strategies for integrating FP into ART services existed at the time of this study (Searing et al. 2008), this project focused on the facilitated referral model after both NACP and RCHS officials indicated this strategy was likely to be the most logistically feasible for Tanzanian CTC settings.
The goal of this project was to develop and test the effectiveness of a facilitated referral model for integrating FP services into HIV CTCs in Tanzania. The primary objective was to test the effectiveness of the facilitated referral intervention on reducing the level of unmet need for contraception among female CTC clients. Secondary objectives included assessing the feasibility of the facilitated referral model and fidelity in delivery of the various components of the model.
Methods
Intervention
The facilitated referral model developed for this project was informed by previous work on facilitated referrals (Winch et al. 2005). The model developed included seven service delivery steps (Figure 1) to systematically screen CTC clients for risk of unintended pregnancy, to provide informed choice counselling on contraceptive options or safer pregnancy, and to more effectively ensure linkages between HIV care and treatment and contraceptive services in facilities with co-located CTC and FP clinics. The model was implemented during a female client’s regular CTC visit and screening was repeated each time she visited the CTC to monitor changes in fertility intentions or risk of unintended pregnancy. Women who were identified as being at risk for an unintended pregnancy were counselled on the FP clinic’s ability to address the needs of women with HIV, provided a written referral to FP services co-located within the same facility and physically accompanied to the FP clinic by CTC staff. In addition to the service delivery steps, the intervention included: FP group education sessions held at the CTC; supportive supervision for both CTC and FP staff; monthly meetings with FP and CTC staff to review progress and address challenges implementing the intervention; a monitoring system that complemented the existing national CTC patient record forms; and job aids to help providers screen and counsel CTC clients on pregnancy risk and safer pregnancy options.
Figure 1.

Service delivery steps of the facilitated referral model.
Study sites
Twelve health facilities supported by TUNAJALI in Iringa and Morogoro regions were purposefully selected based on their high CTC client loads and the co-location of both CTCs and FP clinics within the same facility. Of the 12 facilities, 6 were hospitals and 6 were health centres, including both rural and urban facilities. Both regions have levels of unmet need for contraception among married women comparable to the national average: 22.6% in Morogoro and 26.3% in Iringa (National Bureau of Statistics [Tanzania] and ICF Macro 2011). The HIV prevalence rate among women in Iringa is 18.6% and in Morogoro it is 7.1% (TACAIDS et al. 2008).
Implementation
Each of the 12 participating facilities underwent a site visit in July 2009 with FHI 360, NACP and RCHS staff to introduce the study, discuss implementation of the facilitated referral model and to sign a site agreement finalizing the implementation decisions. In September 2009, 69 CTC and FP providers and supervisors participated in a training led by four MOHSW national FP master trainers who had previously undergone extensive FP and HIV/AIDS care and treatment training. The concurrent, but separate, trainings for CTC and FP providers and supervisors focused on the facilitated referral model, screening for FP need, FP counselling and World Health Organisation (WHO) guidelines for medical eligibility criteria for contraceptive use for women with HIV/AIDS. Upon returning to their respective facilities, participants disseminated the facilitated referral model and FP information with their colleagues, and established a monitoring system to track FP referrals and co-ordinated intra- and inter-clinic meetings to review progress and address the challenges of implementing integrated services.
The intervention was implemented for five months (September 2009 to February 2010) during which two supervision visits were made by RCHS, NACP and FHI 360 staff to the facilities. Utilizing a supervision checklist, the team observed the performance of facility staff, provided corrective and supportive feedback, and helped facilities resolve any problems that arose during the implementation period.
Study design, sample size and participants
A quasi-experimental, pre- and post-test, repeated cross-sectional study was conducted to evaluate the impact of the facilitated referral model. A repeated cross-sectional design was considered appropriate because CTC clients regularly come in for services on a monthly basis. If the intervention was well implemented then unmet need for contraception would decrease across clients at a population level after a few months of intervention implementation.
Eligible study participants were female CTC clients age 18 to 45 years, on or not yet started ART, and had a CD4 count >100 or were WHO clinical stages I, II or III according to their medical record. Sample size calculations indicated that sampling 25 clients from each of the 12 sites at pre-intervention and at post-intervention was sufficient to provide 80% power to detect a 10% decrease in unmet need for contraception using a one-sided test at the 0.05 significance level, assuming 30% unmet need pre-intervention. This calculation further assumed that the correlation between outcomes from two clients in the same site at the same time will be no more than 0.01 and that the correlation between two clients in the same site but at different times will be at least half that. To account for possible data collection errors, we intended to select 27 clients per site per time point. In addition to client interviews, at post-intervention, up to four CTC staff members were interviewed per facility.
Data collection
CTC client participants were recruited during open CTC clinic days which varied by facility from once or twice a week for smaller facilities to four or five times a week for larger facilities. Aided by facility staff, research assistants approached all potentially eligible CTC clients as they left the facility after completing their visit (either after receiving CTC services or after FP services if they sought FP services after seeing their CTC provider). Pre-intervention recruitment information indicated that 72% of approached clients agreed to participate and were interviewed, while 19% of those approached were deemed ineligible, 5% refused to participate and 4% were missed. Recruitment statistics were not gathered at post-intervention but were expected to be similar. Data collection included socio-demographic characteristics, fertility desires, contraceptive use, unmet need for contraception and clients’ CTC and FP experiences related to the seven service delivery steps of the facilitated referral model. CTC providers were approached for participation after completion of all CTC client surveys. Data collection included professional designation, length of service at CTC and experiences related to the intervention. Written informed consent was obtained from all participants.
Study approval
Ethical approvals were obtained from FHI 360’s Protection of Human Subjects Committee and in Tanzania from Muhimbili University of Health and Allied Science’s Senate Research and Publication Committee and the National Institute for Medical Research.
Variable definitions and statistical analysis
The primary study outcome was unmet need for contraception among all female CTC clients. Women were defined as having an unmet need if they were sexually active within the past three months, reported they did not want a child within the next year, and were not currently using a modern method of contraception. Modern methods were defined as injectables, oral pills, intrauterine devices (IUDs), implants, female or male sterilization, and consistent use of male or female condoms. Participants were defined as consistent condom users if they reported using condoms all or most of the time within the past three months. For this study, pregnant women were excluded from the calculation of unmet need.
As the overall level of unmet need was lower than anticipated at pre-intervention, an exploratory subpopulation analysis was undertaken examining the change in proportion of unmet need among only sexually active CTC clients (sex in the past three months), along with the change in modern method use, including consistent condom use, and dual method use.
All analyses were conducted in Stata 10.0 (College Park, Texas, USA). The primary analysis assessed the difference in proportions of female CTC clients with unmet need for contraception from pre-intervention to post-intervention, adjusting for age, number of living children, education, WHO clinical stage, region and facility type. The comparison was made using a linear mixed model that included random effects for facility and the facility by time (pre- vs post-intervention) interaction to control for correlation between responses from women at the same facility, both within and across time periods (Murray 1998). As secondary analyses, similar models were used for comparing between pre- and post-intervention for unmet need, use of a modern method and dual method use among sexually active, non-pregnant women. Because it seemed infeasible that the intervention could possibly increase the level of unmet need, the study analysis plan pre-specified that tests would be conducted using a one-sided 0.05 significance level.
Results
Client results
Characteristics of CTC clients interviewed pre- and post-intervention were similar with no significant differences at the 0.05 level (Table 1). Women were on average 34 years old and over two-thirds were taking antiretroviral drugs. The majority were married or in a regular sexual partnership; half were living with their husband/partner. The mean number of living children was nearly equal between the two groups (2.2 and 2.3, respectively) and 62–67% of the participants did not want another child in the future; few women were currently pregnant. Similar percentages of women reported not being sexually active at pre-intervention (50%) and post-intervention (46%). Reasons for not having sex in the last three months were similar between pre- and post-intervention participants.
Table 1.
Characteristics of female CTC clients at pre-intervention and post-intervention
| Pre- intervention | Post- intervention | |
|---|---|---|
| Characteristics | (n = 323) | (n = 299) |
| Client age (years), mean (range) | 34.5 (18–45) | 33.6 (19–45) |
| Education (highest level attained), % | ||
| No formal education | 12 | 12 |
| Some primary | 78 | 79 |
| Some secondary | 8 | 8 |
| Some higher | 2 | 1 |
| Currently on ART, % | 69 | 70 |
| Relationship status, % | ||
| Married | 46 | 44 |
| Single, has regular sexual partner | 20 | 27 |
| Single, no partner | 34 | 28 |
| Living with husband/partner, % | 49 | 54 |
| Has at least one child, % | 89 | 88 |
| Number of living children, mean (range) | 2.3 (0–9) | 2.2 (0–7) |
| Does not desire another child in the future, % | 67 | 62 |
| Currently pregnant, % | 4 | 3 |
| Not sexually active in the past 3 months, % | 50 | 46 |
| Primary reason for no sexual activity in past 3 months, % | (n = 162) | (n = 138) |
| Not feeling well | 33 | 21 |
| No partner | 22 | 23 |
| Partner is away | 17 | 20 |
| Do not want to infect partner | 15 | 18 |
| Pregnant or recently gave birth | 7 | 7 |
| Partner does not feel well | 4 | 5 |
| Religious beliefs | 3 | 6 |
Note: Percentages may not add up to 100% due to missing data.
Table 2 presents results for the primary objective. Pregnant women were excluded from the primary analysis (n = 14 pre-intervention and n = 8 post-intervention) as were women with missing WHO clinical stage data (n = 3 pre-intervention and n = 0 post-intervention). Among all CTC clients, the adjusted decrease in proportion of unmet need (3%) was not significant (P = 0.103). Among only sexually active CTC clients, the adjusted decrease in proportion of unmet need (8%) was marginally significant (P = 0.052). Secondary analysis showed a significant (P = 0.013) adjusted increase of 12% in the proportion of sexually active CTC clients reporting use of a modern contraceptive method over the intervention period. Dual method use also increased significantly (P = 0.004) with an adjusted increase of 16%.
Table 2.
Unadjusted and adjusted differences in unmet need for contraception and contraceptive use among all non-pregnant CTC clients and among only sexually active CTC clients
| Pre- intervention, % | Post- intervention, % | Unadjusted difference, % | Adjusted difference,a % | One-sided P-valueb | |
|---|---|---|---|---|---|
| All non-pregnant CTC clients | (n = 306) | (n = 291) | |||
| Unmet need | 12 | 8 | −4 | −3 | 0.103 |
| Sexually active, non-pregnant CTC clients | (n = 149) | (n = 156) | |||
| Unmet need | 25 | 15 | −10 | −8 | 0.052 |
| Modern method use | 63 | 77 | +14 | +12 | 0.013 |
| Consistent condom use | 53 | 62 | +9 | ||
| Oral pills | 7 | 17 | +10 | ||
| Injectables | 8 | 16 | +8 | ||
| Implant | 3 | 5 | +2 | ||
| Female sterilization | 1 | 2 | +1 | ||
| Intrauterine device | 1 | 1 | 0 | ||
| Dual method use | 8 | 26 | +18 | +16 | 0.004 |
aMixed regression models adjusted for age, number of living children, education, WHO clinical stage, region and facility type. Model includes random effects for facility and a time-facility interaction. Three non-pregnant pre-intervention women with missing WHO stage data were excluded.
bP-value obtained from the mixed model.
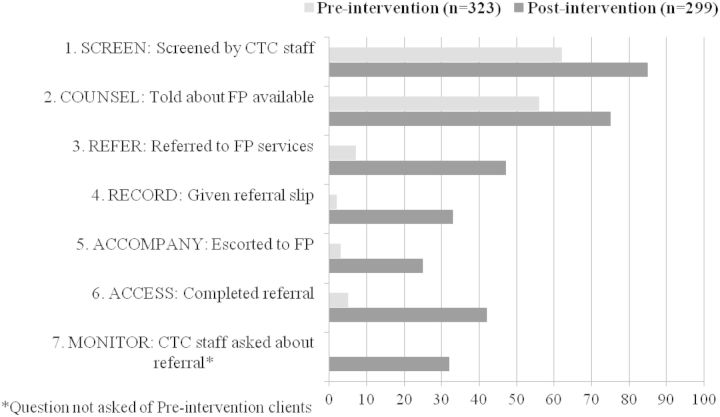
Figure 2 shows the changes in facilitated referral service delivery before and after introduction of the model. Increases were observed for all seven steps of the model for participants at pre-intervention vs post-intervention. Eighty-five percent of all post-intervention clients reported being screened (Step 1) with at least one of the three questions on the screening job aid; however, only 29% of post-intervention clients remembered being asked all three (14% at pre-intervention). For counselling (Step 2), 75% of post-intervention clients said a CTC provider ever discussed contraceptive methods with them, compared with 56% at pre-intervention, and method-specific discussions increased for condoms (52–63%, pre-intervention to post-intervention), pills (26–62%), injectables (26–62%), implants (16–48%), IUDs (12–44%), female sterilization (5–19%) and male sterilization (1–14%).
Figure 2.
Percentages of female CTC clients who report receiving specific facilitated referral steps before and after introduction of the intervention.
Client reports of ever receiving a referral to FP services (Step 3) increased from 7% at pre-intervention to 47% at post-intervention. After the intervention, more clients said they were given written referral forms (Step 4) than at pre-intervention (33% and 2%, respectively), and 25% of post-intervention clients were accompanied (Step 5) during their referrals, compared with 3% at pre-intervention. Additionally, at pre-intervention only 5% of all clients interviewed said they completed their referrals (Step 6), yet nearly half of all post-intervention women (42%) accessed FP services and completed the referrals received from CTC staff. CTC providers monitored the referrals (Step 7) and followed up with their clients as reported by 32% of post-intervention women.
Clients who were referred for FP reported greater frequency of service delivery activities related to their referrals post-intervention compared with pre-intervention (Table 3). More referred clients at post-intervention said they followed through on their referral and went to the FP clinic compared with pre-intervention (91% and 74%, respectively). In addition, post-intervention participants were given referral slips more frequently than pre-intervention clients (71% and 26%) and 10% more post-intervention clients reported being accompanied by CTC staff to the FP clinic. The majority of referred post-intervention clients said they did not have problems with wait-time at the FP clinic. After the intervention, nearly 90% of referred clients said they started a modern contraceptive method from the referral, compared with 65% before the intervention, and 86% reported that their CTC provider knew they had started the method at their next CTC visit.
Table 3.
Percentage of referred CTC clients reporting referral-related service delivery activities at pre-and post-intervention
| Among CTC clients who received a FP referral | Pre- intervention, % (N = 23) | Post- intervention, % (N = 140) |
|---|---|---|
| Referral-related service delivery activities | ||
| Went to FP clinic | 74 | 91 |
| Given referral slip | 26 | 71 |
| Accompanied to FP clinic | 43 | 54 |
| Started modern method at FP clinic | 65 | 87 |
| Did not wait long to see FP provider | n/aa | 71 |
| CTC provider knew client received FP method during return CTC visit | n/aa | 86 |
aQuestion not asked of pre-intervention clients.
Provider results
To explore providers’ experiences with implementing the facilitated referral intervention, 45 CTC staff were interviewed at the 12 study facilities. Half of the providers were nurses and another quarter (22%) were medical doctors or clinical officers, along with health attendants (11%) and counsellors or other staff (16%). The providers worked an average of 2.7 years in the CTC and all directly offered CTC services to clients.
All CTC providers post-intervention agreed with the statement that the integration of FP services into CTC services is a good addition. During the five months in which they implemented the intervention, only 20% of the providers heard complaints from clients about difficulties they faced when trying to access FP services. Specific complaints included the time spent at the FP clinic (9% of all providers), the absence of the FP provider (4%), limited confidentiality (4%), insufficient knowledge about HIV (2%) and a shortage of FP methods (2%). When providers were asked how implementing the intervention impacted their work in the CTC, 60% said the intervention increased their workload and 27% said the new responsibilities took time away from their other CTC services. Nearly two-third of providers (62%) believed their time spent with female clients increased. A shortage of CTC staff was the main challenge providers identified for implementing facilitated referrals, along with the additional workload and the already high number of clients being seen by the CTC each day.
For further perspective on the implementation of the facilitated referral model, providers were asked why they might not carry out several of the specified steps if they indicated that they did not complete each step for every client all of the time. A quarter of CTC providers reported they might not ask clients about their sexual activity or contraceptive use because the client appeared too sick (24% each) or the client is too old to be at risk of pregnancy (24% and 27%, respectively). A client appearing too sick was also the predominant reason (36%) why a provider might not refer a client to FP services. Incomplete screening and referrals were also attributed to a client being pregnant or not interested in referrals. Nine percent of providers reported it was not their responsibility to ask about clients’ sexual activity.
Discussion
This study developed, implemented and tested a seven-step facilitated referral model to serve the FP needs of female HIV care and treatment clients in Tanzania. Although we did not find clear evidence that the facilitated referral intervention appreciably reduced unmet need among all female CTC clients over the intervention period, the primary study outcome, we did observe a trend in this direction in our secondary analysis among sexually active clients. Furthermore, we observed a significant increase in the proportion of sexually active female CTC clients reporting use of a modern contraceptive method. We also saw marked increases in three steps of the model, screening, counselling and referral for FP which, in theory, should have been part of the standard of care for HIV care and treatment clients prior to our intervention. National CTC service guidelines prior to study initiation stated that CTC providers were supposed to address clients’ FP needs with the promotion of condoms and dual method use which could imply the need to screen, counsel and refer clients for non-condom methods. However, the national guidelines did not specify what exactly providers should be doing during a client’s CTC visit.
Following the facilitated referral intervention, providers identified more women with a need for FP and referrals were better documented with referral slips. In addition, at post-intervention more women reported use of oral pills, injectables and implants. This uptake of methods alongside an increase in consistent condom use and dual method use suggests that women were adopting more effective FP methods to use in addition to condom use. However, overall, there were relatively high levels of condom use reported for this population which may, in part, reflect social desirability bias due to being clients in an HIV care and treatment service where condom counselling is routine.
The provider data revealed some logistical challenges for implementation of the intervention, yet this facilitated referral model is the least burdensome integrated service model. Other models would require that CTC providers do all counselling and FP method provision. While provider workloads increased and more time was spent with clients, this was expected given that clients were supposed to receive more comprehensive services.
This study had several limitations. First, due to issues of feasibility we were not able to conduct a clinic-randomized design which would have been preferable to a non-randomized design in order to more confidently attribute observed changes to our intervention; we are unable to rule out other explanations for our observed changes. Second, although a cross-sectional design is suitable for examining changes in unmet need among the overall clinic populations, a cohort design could have been informative because behaviour change (and discussion with partners) takes time and multiple counselling messages from CTC providers about FP may be necessary to see a real effect of the intervention. Third, there is the potential for some bias in the selection of clients; however, we attempted to approach all eligible clients and, at pre-intervention, only 5% refused to participate and 4% were missed due to research assistant unavailability—half at a single larger facility. Finally, the most significant study limitation was our choice of primary outcome measure—unmet need for contraception. The pre-intervention level of unmet need was lower than anticipated which may have made attaining a notable change among all female clients more difficult. Unmet need as a measure of FP/HIV integration effectiveness may not be well suited for HIV care and treatment populations that have high levels of condom use from frequent CTC counselling messages and free condoms. In addition, self-reported condom use likely results in an underestimate of unmet need. The current unmet need algorithm is not sensitive enough to account for women shifting from condoms to a more effective contraceptive method, or for current condom users to adopt dual method use. However, strengths of the study included reporting method-specific changes in contraceptive use and having the intervention implemented in health facilities utilizing existing resources in order to evaluate the intervention within the context of real-world challenges such as understaffed and under-resourced services. The intervention was designed to be applicable to any facility with co-located FP and CTC services and is therefore amenable to scale-up in other Tanzanian facilities that seek to pursue a facilitated model of integration.
This study focused on the essential elements (steps) of facilitated referrals in order to help inform programme development and to help specify where there might be implementation challenges. The data highlight that physically accompanying clients to FP services was particularly challenging while use of referral slips and following up with clients about their referrals were easier to incorporate into practice. Some providers reported not completing certain steps based on their perceptions that clients were not feeling well enough for sexual activity, but Schwartz et al. (2012) found unmet need was greatest in the first year after initiating ART, when women have higher viral loads, lower CD4 counts, and still may appear unwell. Regardless of provider perceptions, all steps of the model need to be completed as part of the systematic service delivery regimen, and integrated into the monitoring and evaluation system. Job aids, patient records, and other forms should be tailored so supervisors can identify client-targets and determine if they are being met thereby holding providers accountable for full intervention implementation.
Determining the best integration strategy for two or more health services depends on a number of factors and not all linkages will make sense in all settings (Kennedy et al. 2010). For HIV–FP integration, others have noted that referral models can be applied in most settings as a minimum standard (Chabikuli et al. 2009) and this model is particularly relevant for settings where services are co-located in the same facilities. As noted by Church and Mayhew (2009), ‘well-organized cross-referral mechanisms may, in fact, be more beneficial and cost-effective than offering multiple services through the same provider or the same facility’. This may be especially true for health care settings that are organized with a variety of vertically-funded programmes (Berer 2004; Druce et al. 2006). However, on the other end of the continuum, full integration (the ‘one-stop-shop’ model) may be efficient in some settings with the necessary staffing and resource availability, and this model is often preferred by clients. Ultimately, selection of an integration model will depend on a number of factors including but not limited to provider time and capacity, organizational structures and funding streams, commodities and procurement processes, and information management. Regardless of the integration model selected, supportive supervision is important along with strong health system policies.
This study provides new evidence on specific, concrete steps needed to implement a facilitated referral model. While we demonstrated the impact of introducing facilitated referrals in a clinical setting seeking to integrate HIV care and treatment and FP services, the steps of the model can be applied to the integration of other health services. Future research on facilitated referrals should focus on evaluating the impact of the specific steps of the model, comparing models with randomized study designs and testing the model with other health services integration approaches.
Authors’ contributions
J.N.B., G.M., M.G. and M.A.W. participated in the conception and design of the study. All authors participated in the design of the programme intervention. M.G., T.W.K., S.N.M. and C.L. implemented the study and oversaw data collection. J.N.B. and M.G. wrote the first draft of the manuscript. M.G. and M.A.W. performed the statistical analysis. All authors were involved in critical revision of the manuscript and read and approved the final manuscript.
Funding
United States Agency for International Development through the Contraceptive and Reproductive Health Technologies Research and Utilization Cooperative Agreement (GPO-A-00-05-000022) and the Preventive Technologies Agreement (GHO-A-00-09-8016-00); the Africa Family Planning and HIV Integration 2007-1423 Fund of Tides Foundation; and the National Center for Advancing Translational Sciences (UL1TR000083). The views expressed in this publication do not necessarily reflect those of FHI 360 or the agencies funding the study.
Conflict of interest
None declared.
References
- Adamchak S, Janowitz B, Liku J, et al. Study of Family Planning and HIV Integrated Services in Five Countries. Research Triangle Park: Family Health International; 2010. [Google Scholar]
- Atun R, de Jongh T, Secci F, Ohiri K, Adeyi O. A systematic review of the evidence on integration of targeted health interventions into health systems. Health Policy and Planning. 2010a;25:1–14. doi: 10.1093/heapol/czp053. [DOI] [PubMed] [Google Scholar]
- Atun R, de Jongh T, Secci F, Ohiri K, Adeyi O. Integration of targeted health interventions into health systems: a conceptual framework for analysis. Health Policy and Planning. 2010b;25:104–11. doi: 10.1093/heapol/czp055. [DOI] [PubMed] [Google Scholar]
- Berer M. HIV/AIDS, sexual and reproductive health: intersections and implications for national programmes. Health Policy and Planning. 2004;19:i62–70. doi: 10.1093/heapol/czh046. [DOI] [PubMed] [Google Scholar]
- Chabikuli NO, Awi DD, Chukwujekwu O, et al. The use of routine monitoring and evaluation systems to assess a referral model of family planning and HIV service integration in Nigeria. AIDS. 2009;23(Suppl. 1):S97–103. doi: 10.1097/01.aids.0000363782.50580.d8. [DOI] [PubMed] [Google Scholar]
- Chibwesha CJ, Li MS, Matoba CK, et al. Modern contraceptive and dual method use among HIV-infected women in Lusaka, Zambia. Infectious Diseases in Obstetrics and Gynegology. 2011;2011:261453. doi: 10.1155/2011/261453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church K, Mayhew SH. Integration of STI and HIV prevention, care, and treatment into family planning services: a review of the literature. Studies in Family Planning. 2009;40:171–86. doi: 10.1111/j.1728-4465.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- Ciampa PJ, Burlison JR, Blevins M, et al. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. Journal of Acquired Immune Deficiency Syndrome. 2011;58:115–9. doi: 10.1097/QAI.0b013e31822149bf. [DOI] [PubMed] [Google Scholar]
- Deloitte. Strengthening the HIV/AIDS Response in Tanzania: Program Leadership for the TUNAJALI (We Care) Program. 2010. http://www.deloitte.com/assets/Dcom-UnitedStates/Local%20Assets/Documents/us_chs_ProgramLeadershipfortheTUNAJALI(We%20Care)_Tanzania_0610.pdf, accessed 24 September 2012. [Google Scholar]
- Deloitte. Tracking HIV Lost to Follow-up Patients: Best Practices and Lessons Learned from the TUNAJALI Project. 2011. 2011. http://globalblogs.deloitte.com/files/dttl_tunajaliproject_full.pdf, accessed 24 September 2012. [Google Scholar]
- Dickinson C, Attawell K, Druce N. Progress on scaling up integrated services for sexual and reproductive health and HIV. Bulletin of the World Health Organization. 2009;87:846–51. doi: 10.2471/BLT.08.059279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druce N, Dickinson C, Attawell K, White AC, Standing H. Strengthening Linkages for Sexual and Reproductive Health, HIV and AIDS: Progress, Barriers and Opportunities. London: DFID Health Resource Centre; 2006. [Google Scholar]
- French RS, Coope CM, Graham A, et al. One stop shop versus collaborative integration: what is the best way of delivering sexual health services? Sexually Transmitted Infections. 2006;82:202–6. doi: 10.1136/sti.2005.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill CJ, Phiri-Mazala G, Guerina N, et al. Effect of training traditional birth attendants on neonatal mortality (Lufwanyama Neonatal Survival Project): randomized controlled study. British Medical Journal. 2011;342:346–55. doi: 10.1136/bmj.d346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GNP+, ICW, IPPF, UNAIDS, UNFPA, WHO and Young Positives. Rapid Assessment Tool for Sexual & Reproductive Health and HIV Linkages: A Generic Guide. 2008. http://data.unaids.org/pub/Manual/2009/2009_rapid_assesment_brochure_en.pdf, accessed 13 March 2012. [Google Scholar]
- Halperin DT, Stover J, Reynolds HW. Benefits and costs of expanding access to family planning programs to women living with HIV. AIDS. 2009;23(Suppl. 1):S123–30. doi: 10.1097/01.aids.0000363785.73450.5a. [DOI] [PubMed] [Google Scholar]
- Kennedy CE, Spaulding AB, Brickley DB, et al. Linking sexual and reproductive health and HIV interventions: a systematic review. Journal of the International AIDS Society. 2010;13:26–35. doi: 10.1186/1758-2652-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp W, Heys J, Jhangri GS, Alibhai A, Rubaale T. Impact of antiretroviral therapy on fertility desires among HIV-infected persons in rural Uganda. Reproductive Health. 2011;6:8–27. doi: 10.1186/1742-4755-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann AS, Gavin L, Galavotti C. The integration of family planning with other health services: a literature review. International Perspectives on Sexual and Reproductive Health. 2010;36:189–96. doi: 10.1363/3618910. [DOI] [PubMed] [Google Scholar]
- Mayhew SH, Lush L, Cleland J, Walt G. Implementing the integration of component services for reproductive health. Studies in Family Planning. 2000;31:151–62. doi: 10.1111/j.1728-4465.2000.00151.x. [DOI] [PubMed] [Google Scholar]
- McCarraher DR, Vance G, Gwarzo U, Taylor D, Chabikuli NO. Changes in contraceptive use following integration of family planning in ART services in Cross River State, Nigeria. Studies in Family Planning. 2011;42:283–90. doi: 10.1111/j.1728-4465.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- Mills A. Vertical vs horizontal health programmes in Africa: idealism, pragmatism, resources and efficiency. Social Science Medicine. 1983;17:1971–81. doi: 10.1016/0277-9536(83)90137-5. [DOI] [PubMed] [Google Scholar]
- Mpangile G, Sima M, van Praag E, Mmbaga V. 2006. Sexual and child-bearing needs of people on ART: the forgotten agenda. Paper presented at the Linking Reproductive Health, Family Planning, and HIV/AIDS in Africa meeting, Addis Ababa, Ethiopia. [Google Scholar]
- Murray DM. Design and Analysis of Group-Randomized Trials. Oxford: Oxford University Press; 1998. [Google Scholar]
- Myer L, Morroni C, Rebe K. Prevalence and determinants of fertility intentions among HIV-infected women and men receiving antiretroviral therapy in South Africa. AIDS Patient Care and STDS. 2007;21:278–85. doi: 10.1089/apc.2006.0108. [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics (NBS) [Tanzania] and ICF Macro. Tanzania Demographic and Health Survey 2010. Dar es Salaam, Tanzania: NBS and ICF Macro; 2011. [Google Scholar]
- Oliff M, Mayaud P, Brugha R, Semakafu AM. Integration reproductive health services in a reforming health sector: the case of Tanzania. Reproductive Health Matters. 2003;11:37–48. [PubMed] [Google Scholar]
- PATH. Convergence of HIV and SRH Services in India: Impacts on and Implications for Key Populations. New Delhi, India: PATH; 2007. [Google Scholar]
- Reynolds HW, Janowitz B, Homan R, Johnson L. The value of contraception to prevent perinatal HIV transmission. Sexually Transmitted Diseases. 2006;33:350–6. doi: 10.1097/01.olq.0000194602.01058.e1. [DOI] [PubMed] [Google Scholar]
- Schwartz SR, Rees H, Mehta S, et al. High incidence of unplanned pregnancy after antiretroviral therapy initiation: findings from a prospective cohort study in South Africa. PLoS One. 2012;7:e36039. doi: 10.1371/journal.pone.0036039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searing H, Farrell B, Gutin S, et al. Evaluation of a Family Planning and Antiretroviral Therapy Integration Pilot in Mbale, Uganda. Evaluation and Research Study No. 13. 2008. New York: EngenderHealth/The ACQUIRE Project; 2008. [Google Scholar]
- Shigayeva A, Atun R, McKee M, Coker R. Health systems, communicable diseases and integration. Health Policy and Planning. 2010;25:i4–20. doi: 10.1093/heapol/czq060. [DOI] [PubMed] [Google Scholar]
- Shlay JC, Mayhugh B, Foster M, et al. Initiating contraception in sexually transmitted disease clinic settings: a randomized trial. American Journal of Obstetrics and Gynecology. 2003;189:473–81. doi: 10.1067/s0002-9378(03)00493-9. [DOI] [PubMed] [Google Scholar]
- Smit JA, Church K, Milford C, Harrison AD, Beksinska ME. Key informant perspectives on policy- and service-level challenges and opportunities for delivering integrated sexual and reproductive health and HIV care in South Africa. BMC Health Services Research. 2012;12:48–55. doi: 10.1186/1472-6963-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding AB, Brickley DB, Kennedy C, et al. Linking family planning with HIV/AIDS interventions: a systematic review of the evidence. AIDS. 2009;23(Suppl. 1):S79–88. doi: 10.1097/01.aids.0000363780.42956.ff. [DOI] [PubMed] [Google Scholar]
- Sweat MD, O'Reilly KR, Schmid GP, Denison J, de Zoysa I. Cost-effectiveness of nevirapine to prevent mother-to-child HIV transmission in eight African countries. AIDS. 2004;18:1661–71. doi: 10.1097/01.aids.0000131353.06784.8f. [DOI] [PubMed] [Google Scholar]
- Sweeney S, Obure CD, Maier CB, et al. Costs and efficiency of integrating HIV/AIDS services with other health services: a systematic review of evidence and experience. Sexually Transmitted Infections. 2012;88:85–99. doi: 10.1136/sextrans-2011-050199. [DOI] [PubMed] [Google Scholar]
- Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS) and Macro International Inc. Tanzania HIV/AIDS and Malaria Indicator Survey 2007-08. Dar es Salaam, Tanzania: TACAIDS, ZAC, NBS, OCGS and Macro International Inc; 2008. [Google Scholar]
- UNAIDS. Joint Action for Results UNAIDS Outcome Framework. 2010. http://data.unaids.org/pub/BaseDocument/2010/jc1713_joint_action_en.pdf, accessed 24 September 2012. [Google Scholar]
- United Nations General Assembly. Resolution 65/277. Political Declaration on HIV/AIDS: Intensifying our Efforts to Eliminate HIV/AIDS. 2011. http://www.unaids.org/en/media/unaids/contentassets/documents/document/2011/06/20110610_un_a-res-65-277_en.pdf, accessed 24 September 2012. [Google Scholar]
- Wanyenze RK, Tumwesigye NM, Kindyomunda R, et al. Uptake of family planning methods and unplanned pregnancies among HIV-infected individuals: a cross-sectional survey among clients at HIV clinics in Uganda. Journal of the International AIDS Society. 2011;14:35. doi: 10.1186/1758-2652-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. PMTCT Strategic Vision 2010-2015: Preventing Mother to Child Transmission of HIV to Reach the UNGASS and Millennium Development Goals. 2010. http://www.who.int/hiv/pub/mtct/strategic_vision.pdf, accessed 24 September 2012. [Google Scholar]
- Wilcher R, Cates W. Reaching the underserved: family planning for women with HIV. Studies in family planning. Studies in Family Planning. 2010;41:125–8. doi: 10.1111/j.1728-4465.2010.00233.x. [DOI] [PubMed] [Google Scholar]
- Winch PJ, Gilroy KE, Wolfheim C, et al. Intervention models for the management of children with signs of pneumonia or malaria by community health workers. Health Policy and Planning. 2005;20:199–212. doi: 10.1093/heapol/czi027. [DOI] [PubMed] [Google Scholar]