Fig. 2.
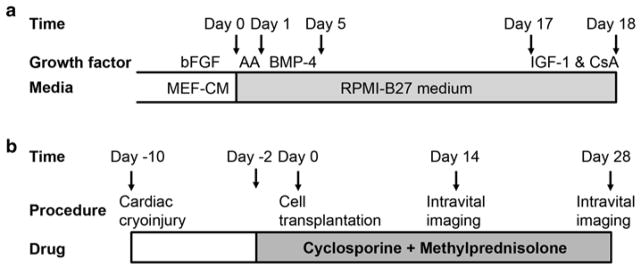
Timelines for the cardiac differentiation of GCaMP+ hESCs and the intravital imaging of GCaMP3+ hESC-CM grafts in injured hearts. (a) GCaMP3+ hESC-CMs are generated using our directed cardiac differentiation protocol, previously described for wild-type hESCs [17] (see Subheading 3.2 for details). In brief, undifferentiated GCaMP3+ hESCs are expanded under standard feeder-free conditions using mouse embryonic fibroblast-conditioned medium (MEF-CM) supplemented with human basic fibroblast growth factor (bFGF). Undifferentiated cultures are then replated to form a dense monolayer in MEF-CM plus bFGF. After a compact monolayer is formed, hESCs are switched to RPMI-B27 medium and serially pulsed with activin A (AA) on day 0 and bone morphogenetic protein-4 (BMP-4) from days 1 to 5. Thereafter, the differentiating cultures are grown in RPMI-B27 medium without any exogenous factors. One day before harvest, cells are heat-shocked and treated with insulin-like growth factor-1 (IGF-1) and cyclosporine (CsA) to improve graft survival. Cells are typically harvested on ~day 18 for cryopreservation or immediate transplantation. (b) To evaluate the electromechanical integration of GCaMP3+ hESC-CM grafts in a subacute model of cardiac injury, guinea pigs undergo a thoracotomy and direct cryoinjury of the left ventricle. 10 days later, a repeat thoracotomy is performed, and GCaMP3+ hESC-CMs are injected into the injured heart. Intravital imaging is typically performed either 14 or 28 days post-transplantation. To avoid graft rejection, animals are treated from day −2 to day 28 post-transplantation with methylprednisolone and CsA
