Abstract
A patient with Mycobacterium abscessus lung disease was mistaken to have pulmonary tuberculosis with airway colonization by the non-tuberculous mycobacterium. Appropriate antibiotics were only given when the patient's signs and symptoms worsened while on anti-tuberculosis therapy. Despite treatment with a combination of antibiotics showing in vitro susceptibility, the pathogen persisted in the respiratory secretions for longer than 6 months and the patient suffered a spontaneous pneumothorax 14 months into treatment. This case illustrates the chronic course of M. abscessus lung infection, the tendency for flare-ups, the inadequacy of current treatment regimens, and the necessity for prolonged patient follow-up.
Keywords: Indolent infection, Mycobacterium abscessus, non-tuberculous mycobacterium, sensitivity test, treatment regimen
Introduction
Non-tuberculous mycobacteria (NTM) are often dismissed as colonizers in the respiratory tract as most of them are environmental organisms with low virulence. Even when they are the etiologic agents, the infections they caused may take months or even years to manifest clinically. Here, we report a case of Mycobacterium abscessus infection to illustrate diagnostic and management difficulties.
Case Report
A 38-year-old woman with scoliosis and Tetralogy of Fallot who had undergone Taussig shunt surgeries in childhood, presented in May 2009 with productive cough, low-grade fever, anorexia, and weight loss. Her chest radiograph (CXR) showed multiple cavities and reticulonodular infiltrates in the right upper lobe. Her sputum was smear-positive for acid-fast bacilli (AFB), but was not cultured for mycobacteria. She was treated for pulmonary tuberculosis (PTB) with ethambutol, isoniazid, rifampicin, and pyrazinamide for 8 weeks followed by rifampicin and isoniazid. However, she continued to have intermittent haemoptysis with one massive episode in September 2009 that required bronchial artery embolization. Her CXR then (Fig. 1) showed increased cavities and infiltrates and her sputum was again AFB positive.
Figure 1.
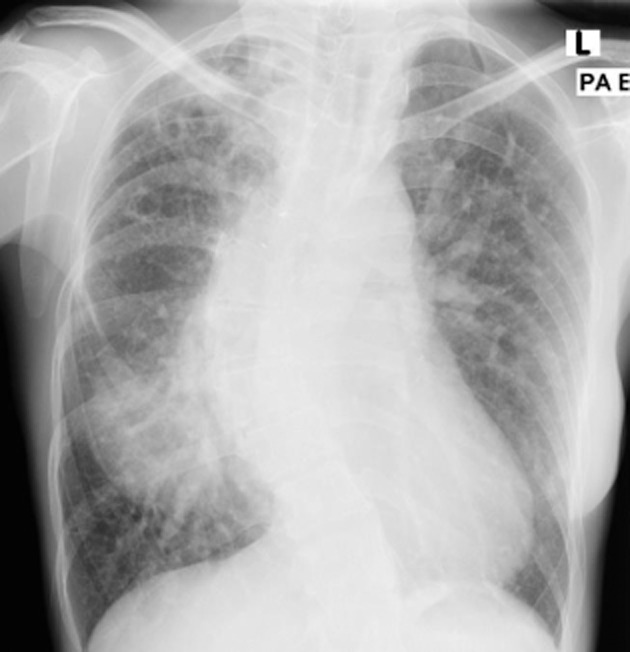
Chest radiograph in September 2009 showed multiple cavities and reticulonodular opacities at right upper zone.
From her bronchoalveolar lavage (BAL) specimen taken in October 2009, M. abscessus was isolated and identified by a reverse line probe hybridization assay (GenoType Mycobacterium CM; Hain Lifescience GmbH, Nehren, Germany). Mycobacterium tuberculosis was not isolated. Mycobacterium abscessus was thought to be a colonizer in the diseased lung and anti-TB chemotherapy was continued until October 2010.
In November 2011, her CXR and computed tomography scan (Fig. 2) showed further enlarged upper lobe cavities associated with increased adjacent lung infiltrates. As her sputum was again culture-positive for M. abscessus, her worsening radiological findings were taken as evidence of chronic M. abscessus infection and she was treated for 8 weeks with intravenous (IV) imipenem 500 mg thrice daily, IV amikacin 15 mg/kg daily, and oral azithromycin 250 mg once daily (as she only weighed 32 kg). Subsequently, treatment was continued with oral azithromycin 250 mg once daily, levofloxacin 500 mg once daily, and doxycycline 100 mg twice daily, for a planned duration of 18 months. In February 2012, a repeat BAL specimen was smear- and culture-negative for mycobacterium. However, in June 2012, after 6 months of combination therapy, her BAL specimen was again positive for M. abscessus. Nevertheless, she continued to receive the same antibiotic combination since she had improved clinically and radiologically.
Figure 2.
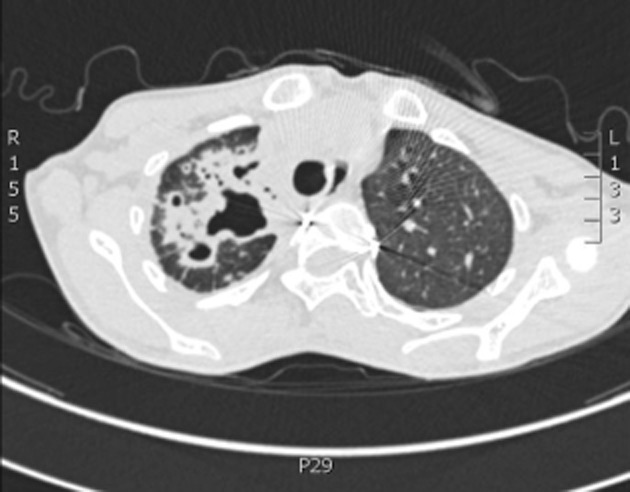
Computed tomography scan of thorax in November 2011 showed enlarging cavities and increased lung infiltrates at right upper lobe.
After 11 months of treatment, a repeat BAL specimen in November 2012 was clear of mycobacterium. She remained well until 13 February 2013 when she developed a spontaneous right-sided pneumothorax which was treated successfully by chest-tube drainage.
The M. abscessus isolated in December 2011 and June 2012 were identified as M. abscessus subsp. massiliense by hsp65 gene sequence analysis. Minimum inhibitory concentrations (MICs) determined with the E-test (AB Biodisk, Solna, Sweden) and interpreted according to Clinical and Laboratory Standards Institute guidelines (NCCLS M24-A 2003) showed both strains to be sensitive to clarithromycin, amikacin, ciprofloxacin, and linezolid (mean MICs of 0.16, 1.75, 0.047, and 0.11 mg/L, respectively), but resistant to imipenem (MIC > 32 mg/L). Doxycycline susceptibility in both strains was indicated by a large inhibition zone diameter (50 mm) in an agar disk diffusion test. A variable number tandem repeat (VNTR) assay showed the two strains belonging to the same VNTR type, indicating persistent colonization by the same strain.
Discussion
NTMs are frequently isolated from respiratory secretions, sometimes concurrently with M. tuberculosis [1]. In our hospital, NTMs account for about 25% of mycobacteria isolated from sputum cultures, of which almost 30% are M. abscessus. It is, however, not always possible to distinguish between infection and colonization. Our patient had clear signs of parenchymal lung disease. Although PTB could be a cause of her presenting symptoms in 2009, this diagnosis was never confirmed by culture and AFB persisted in her sputum even after one and a half years of anti-TB therapy. On the other hand, M. abscessus was isolated three times and her symptoms and signs improved with anti-NTM chemotherapy. Hence, it is possible that the patient had a M. abscessus infection from the beginning rather than a secondary M. abscessus infection complicating PTB.
Combination chemotherapy is required for both empirical and specific treatment of M. abscessus infections because of antibiotic susceptibilities that vary by subspecies and geographical location; and because monotherapy often fail to produce a microbiologic cure [2]. The usual combination comprises clarithromycin or azithromycin plus amikacin and one of the following: fluoroquinolone, imipenem, doxycycline, or linezolid. However, recent publications have highlighted high rates of resistance to some of these antibiotics (55–95% to imipenem [3], >90% to doxycycline [4] and ciprofloxacin [4], and about 50% to linezoid [5]). Clarithromycin resistance ranges from <15% to >70% depending on the subspecies involved [2].
The clinical course of our patient was complicated by an initial misdiagnosis and subsequent dismissal of M. abscessus as an airway colonizer. When she was finally treated for M. abscessus infection, the pathogen persisted for at least 6 months, despite in vitro susceptibility to four of the five antibiotics given. The clearance of M. abscessus after a further 6 months of combination therapy could also be a temporary disease regression as it has been reported that therapy with antibiotics showing in vitro susceptibility often does not produce long-term sputum conversion in patients with M. abscessus lung disease [2]. Hence, our patient will require long-term follow-up and intermittent mycobacterial culture surveillance to rule out the reactivation of this infection.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Funding Statement
The laboratory studies in this case study were done with funding from the Ministry of Higher Education, Malaysia (Grant number LR004/2011A).
References
- 1.Damaraju D, Jamieson F, Chedore P, et al. Isolation of non-tuberculous mycobacteria among patients with pulmonary tuberculosis in Ontario, Canada. Int. J. Tuberc. Lung Dis. 2013;17:676–681. doi: 10.5588/ijtld.12.0684. [DOI] [PubMed] [Google Scholar]
- 2.Bastian S, Veziris N, Roux AL, et al. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob. Agents Chemother. 2011;55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chihara S, Smith G, Petti CA. Carbapenem susceptibility patterns for clinical isolates of Mycobacterium abscessus determined by the E-test method. J. Clin. Microbiol. 2010;48:579–580. doi: 10.1128/JCM.01930-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang SC, Hsueh PR, Lai HC, et al. High prevalence of antimicrobial resistance in rapidly growing Mycobacteria in Taiwan. Antimicrob. Agents Chemother. 2003;47:1958–1962. doi: 10.1128/AAC.47.6.1958-1962.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace RJ, Jr, Brown-Elliott BA, Ward SC, et al. Activities of linezolid against rapidly growing Mycobacteria. Antimicrob. Agents Chemother. 2001;45:764–767. doi: 10.1128/AAC.45.3.764-767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


