Abstract
Key Clinical Message
Traumatic intracranial internal carotid artery dissection is a rare but significant cause of stroke in patients in their forties, leading to high morbidity and mortality. Simultaneous ischemic stroke and optic nerve infarction can occur. Clinical suspicion of dissection is determining in the acute management.
Keywords: Internal carotid artery, optico-cerebral syndrome, traumatic dissection
Introduction
The internal carotid artery dissection (ICAD), as a cause of stroke, is infrequent yet significant, being responsible for 20% of ischemic strokes in patients under 50 years of age [1–4]. It can occur at both the intracraneal and extracranial level. However, the former localization is much less common than the latter [5].
The incidence of ICAD is estimated to be about 1.7 per 100,000 inhabitants per year [6,7], it can be spontaneous or traumatic, the first being common in older individuals of 50 years while the second occurs in younger patients (about 40 years) [8]. ICAD of traumatic origin is rare; its incidence though still unknown, however, is estimated at around 0.08% [9].
The etiology of ICAD is multifactorial, and the pathophysiology is not completely understood. However, there are intrinsic and extrinsic factors that contribute to its development and predispose an individual. Intrinsic susceptibility of an individual is given by the existence of certain anomalies of the arterial wall (fibromuscular dysplasia, dilated aortic root, hyperdistensibility of the arterial wall, or endothelial dysfunction), or by genetic predisposition, less than 2% of cases of dissections have been associated with monogenic connective tissue disease (Ehlers Danlos syndrome, Marfan syndrome, polycystic kidney disease, deficiency of alpha-1 antitrypsin and hereditary hemochromatosis) [4,6,10–15]. The proposed extrinsic factors that act as triggers or predisposing are recent viral infection, hyperhomocysteinemia, and cervical trauma, including both penetrating and nonpenetrating traumas, or even minor mechanical trauma [10,16,17].
There are vascular risk factors connected to ICAD, especially in individuals over 50 years of age. A study showed an association with coronary heart disease (33%), hypertension (57%), and hypercholesterolemia (29%) [8], also mentioned history of smoking (45%) and history of migraine (21%) as risk factors [3,16].
Episodes of stroke in ICAD reflect ischemia caused by the reduction of cerebral blood flow due to stenosis by an intramural hematoma or arterial occlusion by an embolization of a local thrombus, resulting in ischemic stroke or transient ischemic attack (TIA) of the brain or optic nerve [3,4,18–20].
Amaurosis may occur with disease of the carotid artery, but very rarely with simultaneous ipsilateral cerebral stroke [21]. Some medical authors have suggested that artery-to-artery embolism stemming from a thrombus originated in the injured intima is the primary source of stroke. However, other experts speculate that the hemodynamic process (resulting in stenosis) plays a particularly crucial role [3,22,23], the latter mechanism being the less frequent (5%) [3].
Therefore, we examine a case involving permanent visual impairment and contralateral hemiparesia as a consequence of embolism originated of trauma-induced ICAD.
Case Study
A male patient, age 41, arrived at emergency services 30 min after suffering trauma with a blunt object in the cranial and cervical region, which caused loss of consciousness for a few minutes. Subsequently, he went to the hospital. Upon admission vital signs were stable. On physical examination, an abrasion on the right side of the neck and carotid bruit were found. However, in the first hour after trauma, clinical assessment did not detect neurological damage, so the patient was discharged from emergency services.
Forty-eight hours after discharge, the patient presented intense holocranial headache and a loss of visual acuity in the right eye leading to blindness and left motor deficit. Experiencing these symptoms, the patient was returned to the emergency room. On neurological examination, amaurosis of the right eye, afferent pupillary defect, and a normal fundus were found. Hemiparesis, hyperreflexia, and left superficial hemisensory loss were also identified. In addition, left-side Babinski reflex was present. Clinically, stroke was diagnosed and given her clinical picture of posttraumatic stroke, carotid artery injury was suspected.
Tomography allowed observation of a hypodense area located in the caudate nucleus head and the anterior arm of the internal right capsule, extending as far as the frontal ipsilateral region. Magnetic resonance imaging (MRI) demonstrated areas of hyperintensity located in the right striatum nucleus and adjacent corona radiate, which approached the ipsilateral temporo-occipital cortex and fronto-parietal lobe (Figs.1 and 2). These images correspond with an acute stroke localized in the right middle cerebral artery (MCA). Angiography showed significant and eccentric decrease in the size of the supraclinoid segment of the right internal carotid artery, along with moderate stenosis (Figs.3 and 4).
Figure 1.
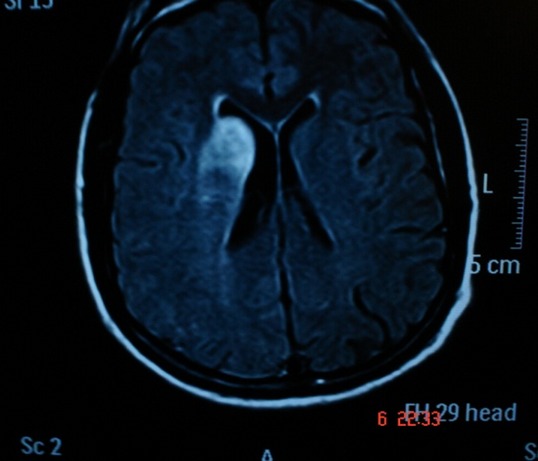
MRI Т1-weighted. Subcortical lesions are localized in the right striatum corpum and adjacent corona radiated.
Figure 2.
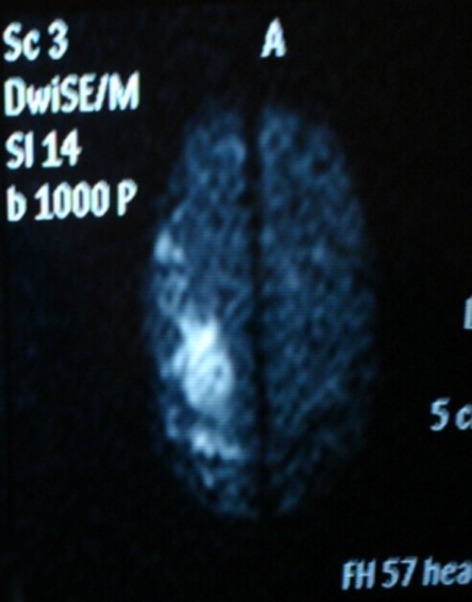
Diffusion-weighted MRI reveals a right cortical hyperintense image that corresponds to an acute cortical infarction.
Figure 3.
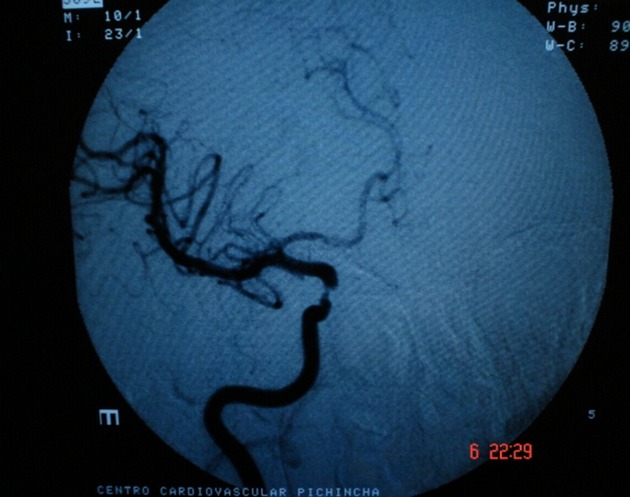
Cerebral angiography of right carotid shows significant and eccentric decrease in the caliber of supraclinoid segment of right internal carotid artery.
Figure 4.
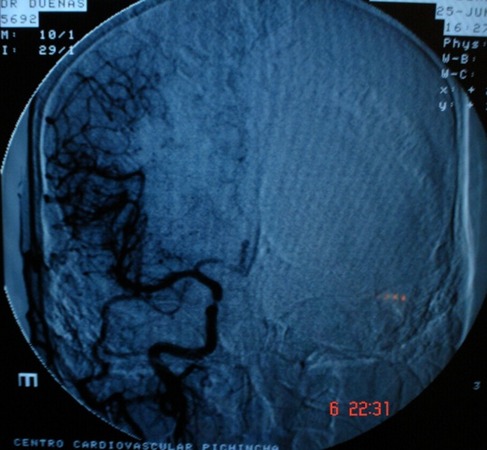
Cerebral angiography of right carotid shows significant and eccentric decrease in the caliber of supraclinoid segment of right internal carotid artery.
The patient was hospitalized for treatment and monitoring. Treatment was started with low-molecular weight heparin, followed by warfarin to achieve an INR value (international normalized ratio) between 2 and 3. The patient continued for 3 months with this medication. At 3 months, a significant functional recovery with a modified Rankin Scale 2 was observed.
Discussion
Traumatic ICAD (TICAD) is a rare and serious cause of embolic stroke in young patients. The basic pathophysiological cause of the dissection is the elongation of the artery produced by a mechanism of hyperextension-rotation or flexion-distraction [24]. Only 10% of cases have immediate symptoms and, unlike our patient, most clinical signs usually occur within the first 24 h of the occurrence of the trauma [25,26].
Traumatic ICAD is suspected and diagnosed when neurological symptoms occur unexpectedly after a trauma. TICAD evolves into stroke in 80% of cases within the first week of the trauma. The common cause of stroke is arterial thrombosis resulting in permanent neurological deficits, with a mortality rate approaching 40% [26].
In general terms, clinical findings are different when traumatic and spontaneous cases of ICAD are compared. With TICAD, the cerebral ischemic symptoms are the most common clinical manifestations, as is seen in the case of our patient. With spontaneous ICAD, unilateral headache (65–68%) and Horner's syndrome (28–41%) are the symptoms most commonly experienced [2,4,22,27,28].
Our patient displayed permanent right monocular blindness and left motor-sensory deficit as a result of ICAD following a trauma. In the clinical course of the patient's illness we can appreciate that the signs and symptoms started within 48 h after the cervical trauma (ICAD). As Biousse's study of 146 cases of extracranial ICAD shows us, the ophthalmological symptoms of patients occurred from the first hour until day 31, whereas patients with ischemic stroke presented symptoms occurring within the first up until the second week [29].
Intracranial ICAD differs from extracranial courses in two different clinical ways: one occurs by way of a subarachnoid hemorrhage and the other presents with headache and/or symptoms associated with ischemic stroke due to cortical stroke, or, as in our case, subcortical stroke, and is produced from arterial occlusion of an embolus (thromboembolic events), or hemodynamic alterations that lead to ischemic stroke in a border zone, which was not observed in the imaging studies in our case (Fig.1) [19,24]. According to the literature, ICAD occurs with ischemic stroke in 80–90% of cases [3,28], TIA in 15–16%, amaurosis fugax in 3%, and ischemic optic neuropathy in 4% [28].
The factors influencing the severity and extent of stroke are collateral circulation and spontaneous recanalization of the artery [30,31]. Through ultrasound of the internal carotid artery, Nedeltchev et al. demonstrated that spontaneous complete arterial recanalization was 16% at 1 month, 50% at 3 months, and 60% at 6–12 months. The occurrence of local symptoms and signs only at presentation were independently associated with complete recanalization [31,32].
The visual disturbance seen in our patient was classified as a case of posterior ischemic optic neuropathy (PION) because of the observed symptoms, including the permanent right amaurosis and normal results of ocular fundus examination; findings were consistent with the data described in studies by Hayreh [33], where 42 patients with PION had amaurosis and normal ocular fundus examination results.
Cases of PION, according to etiology, are divided into three types: arteritic, nonarteritic, and postsurgical. Our patient was categorized as the nonarteritic form, where there is an association with a variety of diseases, among which are cerebrovascular disease, occlusive carotid disease, and ICAD, among many others [34,35]. PION produced by an occlusion of the central retinal artery (CRA) is rare (1%) [27,28,36], and even then, it is in the joint presence of cerebral infarction and PION, as it is in the case of this particular patient. Therefore, there have been no well-documented cases, so far [37].
In regard to the mechanism of infarction, it is necessary to establish whether it is the result of an embolic occlusion or a hemodynamic disorder. Thus, we have conducted an analysis of the literature. Two studies involving a total of 248 and 267 patients with ICAD showed that only 5–11% of brain infarctions were the result of a hemodynamic mechanism, while most were caused by embolic events [2,23]. A retrospective cohort study of 141–143 cases of ICAD reported similar results: thromboembolism (cortical, striatal-capsular, and lacunar) and no hemodynamic infarction was the essential mechanism of stroke. Similar to our case, these studies found that the largest number of infarcts were located in the area of the MCA (99%) (Figs.1 and 2). Five percent were lacunar, and, as in previous studies, 5% of infarctions were hemodynamic in origin [3,12,23].
Generally, it has been established that higher cortical infarcts or those subcortical to 15 mm (as in our case) are embolic in origin [38]. A study that included 40 patients with 65 ICAD found that 52% of infarctions were cortical and 38% were subcortical. All were caused by an artery-to-artery embolism [23].
In addition to MCA infarction, the case of our patient involves other vascular areas, namely, the area of the CRA. In one study, it was found that 0.5% of ischemic stroke patients (3 of 615 cases) showed a neurological deficit similar to our patient's, which they called optico-cerebral syndrome (OCS). They referred to OCS as monocular blindness (amaurosis), accompanied by contralateral motor deficits (hemiparesis). Unlike our case, the three patients identified in this study suffered from a hemodynamic disorder (iatrogenic and postural hypotension), which caused infarctions located in border zone, between the MCA and anterior cerebral artery (ACA) [37]. In accordance with the aforementioned, we can say that our patient had an OCS (due to the presence of amaurosis and contralateral motor-sensory deficit). However, unlike the cases reported in the literature, with the MR images (Figs.1 and 2), we can observe the presence of cortical and subcortical infarction greater than 15 mm in the MCA territory, suggesting an embolic event.
It is well known that the emboli originating from dissection of the carotid artery can occlude the ophthalmic artery without generating any visual deficit, because there is collateral vascular support throughout the posterior ciliary arteries, which prevents ischemia [33,39,40]. Thus far, the permanent monocular blindness in our patient has been attributed to an embolus occluding the CRA [37]. CRA occlusion is caused by an artery-to-artery embolism. However, a hemodynamic condition may also occur [41]. The CRA irrigates the posterior region of optic nerve and, when occluded, produces permanent monocular blindness, yet ocular fundus examination is usually normal, at first [28,33,41]. According to the literature, all patients with nonarteritic PION, carotid artery disease, and history of stroke (with or without carotid artery disease) had significantly increased risk of poor visual test results [42].
In the medical literature, we have found seven cases of ischemic optic neuropathy coinciding with cerebral infarction, three of which were previously discussed and whose etiology, unlike our case, was due to arterial hypotension. In 1990, Rivkin et al. reported a case of PION with ICAD followed 2 days later by a massive stroke. This case, similar to ours, was attributed to an embolus originating from the carotid artery (artery-to-artery embolism). However, ICAD in this particular case, was not caused by trauma, as it was in our patient's case [21,35].
In 1989, Newman et al. reported a case of ICAD that produced a lesion of the MCA and ACA. In contrast to our patient, this case showed edema of the retina and optic nerve head (anterior ischemic optic neuropathy). The ischemic cause attributed in this case was also embolic [21,39]. Bogousslavsky et al. [43] reported two cases of PION with stroke but did not manage to get the details of each case.
In conclusion, according to many published works, we can show that the amaurosis and subcortical infarction of our patient is the product of the embolic occlusion of two vascular areas (ACM and CRA). This is the only reported case of optico-cerebral syndrome (MCA infarction plus NOIP) due to TICAD. The most likely mechanism of infarction was the formation of an embolus (artery to artery), as is substantiated by the imaging studies.
With regard to treatment, there are several options that aim to reduce the extent of neurological deficit and restore cerebral circulation, including thrombolysis, antithrombotic therapy, endovascular management, and surgery (open repair of the carotid artery) [32].
There are no studies on thrombolysis in TICAD. The only data come from a meta-analysis and a multicentric study in spontaneous carotid-vertebral artery dissection (CVAD), and the results are contradictory. Therefore, Zinkstok et al. conducted a systematic search of the literature on intravenous and intraarterial thrombolysis in CVAD. They obtained data of 180 patients, 22 retrospective series, and 14 case reports. Intravenous thrombolysis was performed in 67% of cases and intraarterial thrombolysis was performed in 33%. The follow-up period was 3 months, the rate of symptomatic intracranial hemorrhage was 3.1%, and mortality was 8.1%. Outcomes were dependent on the severity of stroke, 41% of patients had excellent results, thus concluding that the benefit of thrombolysis in CVAD is similar to that observed in other stroke etiologies [44]. In contrast, the CADISP study (cervical artery dissection and ischemic stroke patients) of 660 patients with ischemic stroke due to cervical dissection, where 11% (68 patients) received intravenous thrombolysis, demonstrated that this treatment was associated with no significant increase in intracranial bleeding, and no benefit of thrombolysis was found [45]. Despite these conflicting results, thrombolysis is safe and should be offered to patients with ICAD. Although our patient met criteria for thrombolysis, it was not possible to implement due to a lack of alteplase (rt-PA) at our hospital.
There is controversy over the use of antithrombotic therapy in ICAD. With spontaneous ICAD, the most commonly used regimen is intravenous heparin followed by warfarin for at least 3 months. There is some evidence that antiplatelet agents might be associated with better neurological outcomes in patients with traumatic dissection [32].
Anticoagulation is preferred to antiplatelet agents when there is severe stenosis, arterial occlusion, or pseudoaneurysm, whereas antiplatelet therapy is preferred in cases of large infarcts, intracranial dissections, high risk of bleeding, or inadequate collateral circulation,[32] criteria which were not met in our case. Therefore, it was decided to administer oral anticoagulants even though the available evidence does not show superiority of one therapy over the other.
We can see that a Cochrane review found no randomized controlled studies comparing oral anticoagulants to antiplatelet agents in ICAD. Outcomes are from 36 observational studies that showed no significant difference in mortality or recurrent ischemic stroke. Symptomatic intracranial hemorrhage (0.8%) and extracranial bleeding (1.6%) occurred only in the anticoagulation group but did not reach significant difference [46]. The results of the nonrandomized study in progress at the CADISS arm (cervical artery dissection stroke study) comparing antiplatelet agents and anticoagulants in the prevention of recurrent ischemic stroke in patients with CVAD showed no evidence of superiority between anticoagulation and antiplatelet therapy in terms of stroke recurrence. However, the results of randomized controlled studies are required [47].
To date, there are no randomized controlled studies of the use of endovascular therapy in ICAD. Nevertheless, based on reports of its effectiveness from small case series, it should be recommended in the following circumstances: persistent symptoms despite antithrombotic therapy, secondary aneurysms of dissection that expand, and with compromised cerebral circulation aneurysms,[32] conditions which were not present in our patient.
Conflict of Interest
None declared.
References
- 1.Joseph T, Kandiyil N, Beale D, Tiivas C, Imray CH. A novel treatment for symptomatic carotid dissection. Postgrad. Med. J. 2005;81:e6. doi: 10.1136/pgmj.2004.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartner RW, Arnold M, Baumgartner I, Mosso M, Gönner F, Studer A, et al. Carotid dissection with and without ischemic events: local symptoms and cerebral artery findings. Neurology. 2001;57:827–832. doi: 10.1212/wnl.57.5.827. [DOI] [PubMed] [Google Scholar]
- 3.Benninger DH, Georgiadis D, Kremer C, Studer A, Nedeltchev K, Baumgartner RW. Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke. 2004;35:482–485. doi: 10.1161/01.STR.0000109766.27393.52. [DOI] [PubMed] [Google Scholar]
- 4.Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad. Med. J. 2005;81:383–388. doi: 10.1136/pgmj.2003.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart RG, Easton JD. Dissections. Stroke. 1985;16:925–927. doi: 10.1161/01.str.16.6.925. [DOI] [PubMed] [Google Scholar]
- 6.Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009;8:668–678. doi: 10.1016/S1474-4422(09)70084-5. [DOI] [PubMed] [Google Scholar]
- 7.Lee VH, Brown RD, Jr, Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection.A population-based study. Neurology. 2006;67:1809–1812. doi: 10.1212/01.wnl.0000244486.30455.71. [DOI] [PubMed] [Google Scholar]
- 8.Lleva P, Ahluwalia BS, Marks S, Sahni R, Tenner M, Risucci DA, et al. Traumatic and spontaneous carotid and vertebral artery dissection in a level 1 trauma center. J. Clin. Neurosci. 2012;19:1112–1114. doi: 10.1016/j.jocn.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Davis JW, Holbrook TL, Hoyt DB, Mackersie RC, Field TO, Jr, Shackford SR. Blunt carotid artery disecction incidence, associated injuries, screening, and treatment. J. Trauma. 1990;30:1514–1517. [PubMed] [Google Scholar]
- 10.Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke. 2005;36:1575–1580. doi: 10.1161/01.STR.0000169919.73219.30. [DOI] [PubMed] [Google Scholar]
- 11.Roldán-Valadez E, Corona-Cedillo R, Ruiz-González D, Del Valle R, Herrera-Serrano A, Sánchez-Sánchez JM. Traumatic dissection of extracranial internal carotid artery with middle cerebral artery stroke: imaging diagnosis. Gac. Med. Mex. 2006;142:419–422. [PubMed] [Google Scholar]
- 12.Krings T, Choi IS. The many faces of intracranial arterial dissections. Interv. Neuroradiol. 2010;16:151–160. doi: 10.1177/159101991001600206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanelini MT, Lewkovich GN. An analysis of the etiology cervical artery dissections: 1994 to 2003. J. Manipulative Physiol. Ther. 2005;28:617–622. doi: 10.1016/j.jmpt.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Lin JJ, Chou ML, Lin KL, Wong MC, Wang HS. Cerebral infarct secondary to traumatic carotid artery dissection. Pediatr. Emerg. Care. 2007;23:166–168. doi: 10.1097/PEC.0b013e3180328c24. [DOI] [PubMed] [Google Scholar]
- 15.Debette S, Markus HS. The genetics of cervical artery dissection. A systematic review. Stroke. 2009;40:459–466. doi: 10.1161/STROKEAHA.108.534669. [DOI] [PubMed] [Google Scholar]
- 16.Thomas LC, Rivett DA, Attia JR, Levi CR. Risk factors and clinical presentation of craniocervical arterial dissection: A prospective study. BMC Musculoskelet. Disord. 2012;13:164. doi: 10.1186/1471-2474-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Micheli S, Paciaroni M, Corea F, Agnelli G, Zampolini M, Caso V. Cervical artery dissection: emerging risk factors. Open Neurol. J. 2010;4:50–55. doi: 10.2174/1874205X01004010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono H, Nakatomi H, Tsutsumi K, Inoue T, Teraoka A, Yoshimoto Y, et al. Symptomatic recurrence of intracranial arterial dissections follow-up study of 143 consecutive cases and pathological investigation. Stroke. 2013;44:126–131. doi: 10.1161/STROKEAHA.112.670745. [DOI] [PubMed] [Google Scholar]
- 19.Schevink WI. Spontaneous dissection of the carotid and vertebral arteries. N. Engl. J. Med. 2001;344:898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 20.Redekop GJ. Extracranial carotid and vertebral artery dissection: a review. Can. J. Neurol. Sci. 2008;35:146–152. doi: 10.1017/s0317167100008556. [DOI] [PubMed] [Google Scholar]
- 21.Biousse V, Schaison M, Touboul PJ, D'Anglejan-Chatillon J, Bousser MG. Ischemic optic neuropathy associated with internal carotid artery dissection. Arch. Neurol. 1998;55:715–719. doi: 10.1001/archneur.55.5.715. [DOI] [PubMed] [Google Scholar]
- 22.Engelter ST, Brandt T, Debette S, Caso V, Lichy C, Pezzini A, et al. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38:2605–2611. doi: 10.1161/STROKEAHA.107.489666. [DOI] [PubMed] [Google Scholar]
- 23.Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29:2646–2648. doi: 10.1161/01.str.29.12.2646. [DOI] [PubMed] [Google Scholar]
- 24.Yang ST, Huang YC, Chuang CC, Hsu PW. Traumatic internal carotid artery dissection. J. Clin. Neurosci. 2006;13:123–128. doi: 10.1016/j.jocn.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Sasser PL, Stein MA, Johnson JK. Blunt carotid artery trauma: diagnosis and management. Contemp. Surg. 1992;41:55–59. [Google Scholar]
- 26.Bayır A, Aydoğdu Kıreşi D, Söylemez A, Demirci O. Cerebral infarction caused by traumatic carotid artery dissection. Ulus. Travma. Acil. Cerrahi. Derg. 2012;18:347–350. doi: 10.5505/tjtes.2012.66900. [DOI] [PubMed] [Google Scholar]
- 27.Beletsky V, Norris JW. Spontaneous dissection of the carotid and vertebral arteries. N. Engl. J. Med. 2001;345:467. [PubMed] [Google Scholar]
- 28.Baumgartner RW, Bogousslavsky J. Clinical manifestations of carotid dissection. Front Neurol. Neurosci. 2005;20:70–76. doi: 10.1159/000088151. [DOI] [PubMed] [Google Scholar]
- 29.Biousse V, Touboul PJ, D′Anglejan-Chatillon J, Lévy C, Schaison M, Bousser MG. Ophtalmologic manifestations of internal carotid artery dissection. Am. J. Ophtalmol. 1998;126:565–577. doi: 10.1016/s0002-9394(98)00136-6. [DOI] [PubMed] [Google Scholar]
- 30.Silvestrini M, Altamura C, Cerqua R, Pedone C, Balucani C, Luzzi S, et al. Early activation of intracranial collateral vessels influences the outcome of spontaneous internal carotid arterydissection. Stroke. 2011;42:139–143. doi: 10.1161/STROKEAHA.110.595843. [DOI] [PubMed] [Google Scholar]
- 31.Nedeltchev K, Bickel S, Arnold M, Sarikaya H, Georgiadis D, Sturzenegger M, et al. Recanalizati on of spontaneous carotid artery dissection. Stroke. 2009;40:499–504. doi: 10.1161/STROKEAHA.108.519694. [DOI] [PubMed] [Google Scholar]
- 32.Mohan IV. Current optimal assessment and management of carotid and vertebral spontaneous and traumatic dissection. Angiology. 2013;11:10. doi: 10.1177/0003319712475154. [DOI] [PubMed] [Google Scholar]
- 33.Hayreh SS. Posterior ischaemic optic neuropathy: clinical features, pathogenesis, and management. Eye (Lond) 2004;18:1188–1206. doi: 10.1038/sj.eye.6701562. [DOI] [PubMed] [Google Scholar]
- 34.Tsai R-K, Sun C-Y. Spontaneous dissection of internal carotid artery presenting as isolated posterior ischaemic optic neuropathy. Br. J. Ophthalmol. 1997;81:513–517. doi: 10.1136/bjo.81.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivkin MJ, Hedges TR, 3rd, Logigian EL. Carotid dissection as posterior ischemic optic neuropathy. Neurology. 1990;40:1469. doi: 10.1212/wnl.40.9.1469. [DOI] [PubMed] [Google Scholar]
- 36.Koch S, Lorenzo D, Rabinstein AA, Lam B. Ischemic optic neuropathy and carotid dissection. Neurology. 2005;64:827. doi: 10.1212/01.WNL.0000145840.79056.07. [DOI] [PubMed] [Google Scholar]
- 37.Bogousslavsky J, Regli F, Zografos L, Uske A. Optico-cerebral syndrome: simultaneous hemodynamic infarction of optic nerve and brain. Neurology. 1987;37:263–268. doi: 10.1212/wnl.37.2.263. [DOI] [PubMed] [Google Scholar]
- 38.Horowitz DR, Tuhrim S. Stroke mechanisms and clinical presentation in Large subcortical infarctions. Neurology. 1997;49:1538–1541. doi: 10.1212/wnl.49.6.1538. [DOI] [PubMed] [Google Scholar]
- 39.Newman NJ, Kline LB, Leifer D, Lessell S. Ocular stroke and carotid artery dissection. Neurology. 1989;39:1462–1464. doi: 10.1212/wnl.39.11.1462. [DOI] [PubMed] [Google Scholar]
- 40.Williams EL, Hart WM, Jr, Tempelhoff R. Postoperative ischemic optic neuropathy. Anesth. Analg. 1995;80:1018–1029. doi: 10.1097/00000539-199505000-00029. [DOI] [PubMed] [Google Scholar]
- 41.Lee SK, Kwon SU, Ahn H, Kim JS. Acute isolated monocular blindness and painless carotid artery dissection. Neurology. 1999;53:1155–1156. doi: 10.1212/wnl.53.5.1155. [DOI] [PubMed] [Google Scholar]
- 42.Sadda SR, Nee M, Miller NR, Biousse V, Newman NJ, Kouzis A. Clinical spectrum of posterior ischemic optic neuropathy. Am. J. Ophthalmol. 2001;132:743–750. doi: 10.1016/s0002-9394(01)01199-0. [DOI] [PubMed] [Google Scholar]
- 43.Bogousslavsky J, Despland PA, Regli F. Spontaneous carotid dissection with acute stroke. Arch. Neurol. 1987;44:137–140. doi: 10.1001/archneur.1987.00520140009010. [DOI] [PubMed] [Google Scholar]
- 44.Zinkstok SM, Vergouwen MD, Engelter ST, Lyrer PA, Bonati LH, Arnold M, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42:2515–2520. doi: 10.1161/STROKEAHA.111.617282. [DOI] [PubMed] [Google Scholar]
- 45.Engelter ST, Dallongeville J, Kloss M, Metso TM, Leys D, Brandt T, et al. Thrombolysis in cervical artery dissection–data from the Cervical Artery Dissection and Ischaemic Stroke Patients (CADISP) database. Eur. J. Neurol. 2012;19:1199–1206. doi: 10.1111/j.1468-1331.2012.03704.x. [DOI] [PubMed] [Google Scholar]
- 46.Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst. Rev. 2010;(10):CD000255. doi: 10.1002/14651858.CD000255. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy F, Lanfranconi S, Hicks C, Reid J, Gompertz P, Price C, et al. Antiplatelets vs anticoagulation for dissection: CADISS non randomized arm and meta-analysis. Neurology. 2012;79:686–689. doi: 10.1212/WNL.0b013e318264e36b. [DOI] [PubMed] [Google Scholar]


