Abstract
Key Clinical Message
Community-acquired Klebsiella pneumoniae primary liver abscess (KLA) has been emerging worldwide over the past two decades and with high incidence in Asia. The presence of specific virulence characteristics is a risk factor for a syndrome with metastatic complications. This report signals an increasing emergence in Northern Europe.
Keywords: Community-acquired infections, K1, Klebsiella pneumoniae, liver abscess
Introduction
Klebsiella pneumoniae primary liver abscess (KLA) has occurred with increasing incidence in Asia since the early 1980s and is now endemic in Taiwan [1]. A specific syndrome with septicemia and metastatic spread to eyes, meninges, or brain may occur and is associated with highly virulent strains of K. pneumoniae [2]. KLA is considered a rare disease in Western countries, but in the past decade there has been an increase in the number of reported cases from Europe and North America [3–14]. As of 2013, only one case of this emerging infectious entity (a patient of Asian descent) in Scandinavia has been published [11]. We report the first three cases of KLA in Norway, all of whom were admitted to a single University hospital between 2008 and 2011.
Case Reports
Major patient characteristics are presented in the Table1.
Table 1.
Major features of the cases
| Characteristics | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Gender/age (years) | Female 44 | Male 53 | Female 40 |
| Origin | Thailand | West Africa | West Africa |
| Time since visit to native country | 8 months | 4 years | 10 months |
| Comorbidities | None | Hypertension | Diabetes |
| Preadmission symptoms | Fever, abdominal pain | Fever, abdominal pain, rigors, dyspnea | Fever, rigors, polydipsia, polyuria, weight loss |
| Temperature (°C) | 38.3 | 40.2 | 39.1 |
| WBC count, 109/L | 12.4 | 5.9–14.0 | 7.1 |
| CRP (mg/L) | 320 | 80–200 | 150 |
| ALT (U/L) | 113 | 46–170 | 33 |
| CT scan | Solitary hepatic abscess | Solitary hepatic abscess | Multiple hepatic abscesses |
| Positive culture(s) for K. pneumoniae1 | Abscess | Blood | Blood |
| LOS, days | 19 | 12 | 6 |
WBC, white blood cell count; CRP, C-reactive protein; ALT, alanine transaminase; AST, aspartate transaminase; K. pneumoniae, Klebsiella pneumoniae; LOS, length of hospital stay.
All isolates were serotype K1, positive for rmpA, aerobactin, kfu, and allS. All strains belonged to MLST 23. None of the patients had extrahepatic manifestations.
Case 1
A 44-year-old Norwegian female of Thai descent presented with a 6 day history of fever and malaise as well as 3 days with constant abdominal pain in the right upper quadrant. She also reported mild diarrhea, dry cough, and dyspnea.
Her past medical history was unremarkable. She had visited Thailand 8 months earlier. Findings on physical examination included fever of 38.3°C, tachycardia 102/min, tenderness in the right upper quadrant without rebound or guarding. She had no signs of jaundice.
Laboratory findings included a white blood cell count of 12.4 × 109/L, C-reactive protein (CRP) 320 mg/L, slightly elevated total bilirubin 29 μmol/L, alkaline phosphatase 390 U/L, aspartate transaminase (AST) 228 U/L, alanine transaminase (ALT) 113 U/L, albumin 31 g/L, and s-glucose 5.3 mmol/L. Electrolytes and renal function tests were within normal range.
The patient was initially treated empirically with intravenous ciprofloxacin and clindamycin. Ciprofloxacin was rapidly switched to intravenous cefuroxime due to an allergic reaction. Blood cultures were negative.
A right upper quadrant ultrasound was negative for biliary obstruction, but revealed changes in liver parenchyma. CT scan showed a 7.3 × 7.5 cm inhomogeneous abscess in liver segment 6 (Fig.1). Entamoeba histolytica serology was negative. Ultrasound-guided drainage of the abscess yielded purulent fluid, which grew K. pneumoniae, sensitive to all antibiotics except ampicillin. A therapeutic pigtail catheter was inserted, and antibiotics were changed to piperacillin–tazobactam. She was discharged after 19 days, and treated with oral ciprofloxacin (i.e., ciprofloxacin 500 mg bid) for another 4 weeks experiencing no allergic reaction. Follow-up CT scan after treatment showed complete abscess resolution.
Figure 1.
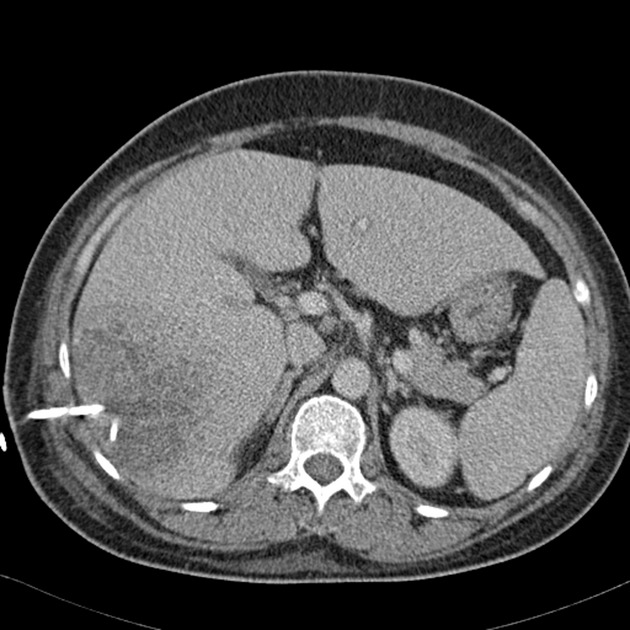
Abdominal CT scan showed a 7.3 × 7.5 cm inhomogeneous abscess in liver segment 6. A therapeutic pigtail catheter was inserted.
Case 2
A 53-year-old male with a past medical history of hypertension developed acute severe malaise with fever and rigors. He also reported thin defecation and intermittent cramp-like abdominal pain accompanied by dyspnea.
The patient was born in West Africa, but had lived many years in Norway. He had not been traveling for the last 4 years. He was in severe distress with a temperature of 40.2°C, but only mild epigastric tenderness was found by thorough physical examination. A urine strip test was positive for leukocytes, protein, blood, and nitritis. Sepsis treatment regimen with parenteral penicillin and gentamicin was initiated. The patient was quickly stabilized.
His white blood cell count was 5.9 × 109/L at admission, rising to 14.0 × 109/L the next morning. He was slightly thrombocytopenic 95 × 109/L and CRP increased rapidly from 80 to 200 mg/L. Liver enzyme values were normal at admission, rising to elevated levels during the first days of hospitalization as he developed signs of jaundice. S-glucose was 6.4 mmol/L.
Klebsiella pneumoniae resistant only to ampicillin grew in four of the four blood cultures. Urine cultures were negative. Ultrasound examination showed a nonobstructive biliary system. An abdominal CT scan revealed a 4 × 4 cm intrahepatic abscess in segment 7. Due to the risks associated with an invasive procedure on this localization, it was considered that such intervention could not be justified. Antibiotics were changed to intravenous cefotaxime and metronidazole, which led to rapid clinical improvement. Because percutaneous drainage was not possible, a 6-week course of oral high-dose ciprofloxacin (i.e., ciprofloxacin 750 mg bid) was given. Despite bacteremia, there were no signs of extrahepatic manifestations. A CT scan 10 days after presentation showed initiating abscess resolution. Follow-up CT scan 4 months later showed complete resolution of the abscess.
Case 3
A 40-year-old female presented with a 6-day history of fever, rigors, malaise, myalgia, anorexia, and weight loss. She also reported polydipsia and polyuria, but no other suspicious symptoms of urinary tract infection or any symptoms suggestive of abdominal involvement. She migrated from West Africa 10 months earlier, and had not been traveling since. Her past medical history was unremarkable.
She was in mild distress with a temperature of 39.1°C and ketonia-smelling breath. A new heart murmur was detected. She had no signs of abdominal tenderness or distension, jaundice, lymphadenopathy, or organomegaly.
Her white blood cell count was 7.1 × 109/L, CRP 150 mg/L, total bilirubin 9 μmol/L, alkaline phosphatase 209 U/L, AST 42 U/L, ALT 33 U/L, s-kalium 4.3 mmol/L, and s-glucose 23.8 mmol/L. Urine strip test showed glucosuria and ketonuria, cultures were negative. Treatment regimen for hyperglycemia was initiated.
Endocarditis was ruled out on the basis of Duke's criteria. Klebsiella pneumoniae resistant only to ampicillin grew in one of the four blood cultures and intravenous cefotaxime was initiated. On the third day of hospitalization, she had continued fever and tachycardia. A physical examination now revealed tenderness in the right upper quadrant. The following ultrasound and CT scan demonstrated multiple hepatic abscesses.
Because none of the abscesses were larger than 5 cm, percutaneous drainage was not performed. There were no signs of extrahepatic infectious foci. Antibiotics were continued with combined intravenous cefotaxime and metronidazole.
She responded well, and was further treated as an outpatient with oral high-dose ciprofloxacin for 4 weeks. Imaging and clinical examination 6 weeks after discharge showed no abnormalities (Table1).
Results/Bacteriology
Virulence characterization was performed retrospectively at the International Escherichia coli and Klebsiella Reference Centre (WHO), Statens Serum Institut and revealed that the isolates belonged to the K1 serotype which was confirmed by detection of the K1 serotype-specific gene magA and expressed the hypermucoviscous phenotype as shown by the formation of mucoviscous strings when a loop was passed through a colony (string test) (Fig.2) [15]. Furthermore, all isolates were positive for all four virulence genes rmpA, aerobactin, kfu, and allS as revealed by polymerase chain reaction using specific primers [2]. Multilocus sequence typing (MLST) revealed that all strains belonged to sequence type (ST) 23.
Figure 2.

String test. Formation of mucoviscous strings when a loop is passed through a colony.
Discussion
A distinctive form of community-acquired primary liver abscess with bacteremia and metastatic infection caused by K. pneumoniae has been well-known in Taiwan and other Asian countries for two decades [16,17]. Although E. coli and polymicrobial infection have been considered the predominant cause of pyogenic liver abscesses in Western countries, K. pneumoniae was reported from the United States in 2004 to be increasingly recovered, especially in Asian patients [18]. Primary liver abscess and bacteremia were later connected to the Taiwanese syndrome associated with the highly virulent hypermucoviscous phenotype of capsular serotype K1. The first cases from North America were reported in 2005 and 2007 [3,9]. In recent years, cases from Argentina, South Africa, Belgium, Spain, France, Ireland, and Sweden have been reported, indicating a global dissemination of these strains causing invasive liver abscesses in both Asian and non-Asian patients [4,5,7,11,12,14]. A Korean study provided evidence for clonal dissemination by sequence typing of the serotype K1 K. pneumoniae invasive isolates from liver abscesses, showing that these strains were genotypically related and belonged to ST 23 [19]. Recently, both an Argentinian and a French study also reported isolates belonging to ST 23, suggesting its global dissemination [4,12]. Our results showed that the isolates exhibited similar characteristics as the highly virulent K. pneumoniae isolates associated with invasive infections in Asia. All isolates belonged to ST 23 supporting the theory of global dissemination. Two of our patients were of West African origin from where there are no published cases of KLA.
The clinical manifestations of KLA are similar to those of pyogenic liver abscesses caused by other etiologies. Although rare, the highly virulent strains of K. pneumoniae are more prone to develop bacteremia and metastatic complications that occur in 12–13% of the cases, with eye, meninges, and brain as the most common sites [2,16]. No specific investigations (e.g., CT or ocular examination) were performed to rule out such complications in our patients. However, they were all followed up a long period after hospital discharge without showing any symptoms or signs that would indicate this.
The pathogenetic mechanisms of KLA remain unclear, although several virulence factors of the invasive strains have been described. Capsular serotype K1 of a characteristic hypermucoviscous phenotype has been reported as a major virulence factor of K. pneumoniae invasiveness causing the syndrome of primary liver abscess and septic metastatic complications [2,20]. These hypermucoviscous strains also demonstrate high resistance to human serum and phagocytosis killing [15]. Four additional virulence genes, the two plasmid encoded rmpA (regulator of mucoid phenotype) and aerobactin (an iron siderophore), as well as two chromosomal encoded virulence genes, allS (associated with allatonin metabolism) and kfu (encoding an iron uptake system), have also been proven to be important virulence determinants [17,21]. Although rare in other clinical isolates from Western countries, K1 is the most common serotype isolated from patients with KLA [20,22].
Diabetes mellitus or impaired fasting glucose is a major risk factor for primary KLA and the frequency of diabetes among patients with KLA has been reported as high as 78% [20]. A case–control study reported that patients with diabetes mellitus had a statistically significant 2.1-fold increased risk of K1 serotype KLA than of non-Klebsiella liver abscess [23]. Poor glycemic control plays a role in impairing neutrophil phagocytosis of these K1 serotype strains which partially can explain these differences [24]. However, it is debated whether diabetes is an independent risk factor for metastatic infection [2,16,17,20]. Of the two bacteremic cases, one was diabetic and the other non-diabetic, thus confirming that these strains are virulent enough to cause severe invasive infection even in a healthy host.
Although the present cases and other reports from Western countries address a global spread of the infection, most patients have been of Asian origin [3,6,8–11,14,18]. Ethnicity and genetic susceptibility may partially explain this pattern. However, earlier colonization in the patients’ home countries has also been suggested as an alternative explanation for the differences in global epidemiology of KLA [13]. A recent Korean study examining fecal carriage of the serotype K1 K. pneumoniae ST 23 demonstrated that the isolates from intestinal carriers and liver abscess patients were closely related genotypically, which support this explanation [25]. Alternatively, some patients may have been infected in their social surroundings by someone with a more recent traveling history. This could be the case for one of our patient from West Africa who had not been abroad for 4 years.
Principles of diagnosis are similar to those of other causes to pyogenic liver abscess, including thorough history, clinical examination, radiographic imaging, and laboratory investigation. Serologic tests are useful in such cases. If possible, purulent material from needle aspiration should be Gram stained and cultured for aerobic and anaerobic bacteria. Underlying hepatobiliary- or pancreatic disease must be considered in general. Risk factors for KLA, such as diabetes and geographic origin, must be assessed in particular by the clinician for early diagnosis. This will be guided by bacterial identification and a simple string test can easily detect the hypermucoviscous phenotype for confirmation [15]. Rapid detection of the virulent K1 serotype will be most helpful in diagnosis and treatment to decrease the risk of severe metastatic infections, as well as in epidemiological studies [26].
Community-acquired KLA isolates rarely produce extended-spectrum β-lactamases (ESBL) [27]. In Norway, the prevalence of ESBL-producing K. pneumoniae isolates in blood cultures is 2.9% [28].
Treatment of KLA requires parenteral antibiotic therapy combined with percutaneous drainage when possible. Drainage should follow the same guidelines as for pyogenic liver abscesses of other causes [29].
Initial empirical antibiotic treatment should be guided by local bacterial resistance patterns and subsequently be tailored to culture- and antibiotic susceptibility results. Norwegian guidelines for hepatic abscess treatment recommend intravenous piperacillin–tazobactam or ceftriaxone and metronidazole for 10–14 days until clinical improvement, followed by oral ciprofloxacin and metronidazole for a total treatment length of 4–6 weeks. Longer courses of treatment may be warranted for patients requiring subsequent drainage procedures or with persistent radiographic evidence of abscess. In general, treatment should be continued until follow-up CT imaging demonstrates complete or near complete resolution of the abscess cavity.
The prognosis of KLA is good if early and adequate treatment is given. Mortality rates range from 2.8% to 11.3% and relapse rates from 4.4% to 6.5% [2,16,30]. Although the mortality rate is relatively low, metastatic infection may cause considerable optic and neurologic disabilities [2,16].
This report presents the first three published cases of hypermucoviscous serotype K1 K. pneumoniae liver abscess in Norway. All cases were presented to a single University hospital, which may signal an increasing emergence in Northern Europe. Awareness by clinicians and public health officials could allow early detection and optimal management of this condition which also affects non-Asians. Further geographic dissemination of hypermucoviscous K1 K. pneumoniae strains of ST23 is likely and a rising number of cases from other geographic regions indicate that this is a globally emerging infectious disease.
Acknowledgments
We would like to thank Arne N. Eskesen, Hedda von der Lippe, and Håvard Aamodt (Department of Infectious Diseases, Akershus University Hospital, Lørenskog, Norway) for the clinical collaboration.
Conflict of Interest
None declared.
References
- 1.Tsai FC, Huang YT, Chang LY, Wang JT. Pyogenic liver abscess as endemic disease, Taiwan. Emerg. Infect. Dis. 2008;14:1592–1600. doi: 10.3201/eid1410.071254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin. Infect. Dis. 2007;45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 3.Fang FC, Sandler N, Libby SJ. Liver abscess caused by magA+ Klebsiella pneumoniae in North America. J. Clin. Microbiol. 2005;43:991–992. doi: 10.1128/JCM.43.2.991-992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decre D, Verdet C, Emirian A, Le GT, Petit JC, Offenstadt G, et al. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J. Clin. Microbiol. 2011;49:3012–3014. doi: 10.1128/JCM.00676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karama EM, Willermain F, Janssens X, Claus M, Van den Wijngaert S, Wang JT, et al. Endogenous endophthalmitis complicating Klebsiella pneumoniae liver abscess in Europe: case report. Int. Ophthalmol. 2008;28:111–113. doi: 10.1007/s10792-007-9111-4. [DOI] [PubMed] [Google Scholar]
- 6.Keynan Y, Karlowsky JA, Walus T, Rubinstein E. Pyogenic liver abscess caused by hypermucoviscous Klebsiella pneumoniae. Scand. J. Infect. Dis. 2007;39:828–830. doi: 10.1080/00365540701266763. [DOI] [PubMed] [Google Scholar]
- 7.Gomez C, Broseta A, Otero JR, Chaves F. Primary pyogenic liver abscess caused by magA+ Klebsiella pneumoniae in Spain. Clin. Microbiol. Newsl. 2007;29:100–102. [Google Scholar]
- 8.Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am. J. Gastroenterol. 2005;100:322–331. doi: 10.1111/j.1572-0241.2005.40310.x. [DOI] [PubMed] [Google Scholar]
- 9.Nadasy KA, Domiati-Saad R, Tribble MA. Invasive Klebsiella pneumoniae syndrome in North America. Clin. Infect. Dis. 2007;45:e25–e28. doi: 10.1086/519424. [DOI] [PubMed] [Google Scholar]
- 10.Rivero A, Gomez E, Alland D, Huang DB, Chiang T. K2 serotype Klebsiella pneumoniae causing a liver abscess associated with infective endocarditis. J. Clin. Microbiol. 2010;48:639–641. doi: 10.1128/JCM.01779-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobirk SK, Struve C, Jacobsson SG. Primary Klebsiella pneumoniae liver abscess with metastatic spread to lung and eye, a North-European case report of an emerging syndrome. Open Microbiol. J. 2010;4:5–7. doi: 10.2174/1874285801004010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vila A, Cassata A, Pagella H, Amadio C, Yeh KM, Chang FY, et al. Appearance of Klebsiella pneumoniae liver abscess syndrome in Argentina: case report and review of molecular mechanisms of pathogenesis. Open Microbiol. J. 2011;5:107–113. doi: 10.2174/1874285801105010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCabe R, Lambert L, Frazee B. Invasive Klebsiella pneumoniae infections, California, USA. Emerg. Infect. Dis. 2010;16:1490–1491. doi: 10.3201/eid1609.100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore R, O'Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection. 2013;41:681–686. doi: 10.1007/s15010-013-0408-0. [DOI] [PubMed] [Google Scholar]
- 15.Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med. 2004;199:697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wang JH, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 1998;26:1434–1438. doi: 10.1086/516369. [DOI] [PubMed] [Google Scholar]
- 17.Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von GA, et al. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg. Infect. Dis. 2007;13:986–993. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin. Infect. Dis. 2004;39:1654–1659. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 19.Chung DR, Lee HR, Lee SS, Kim SW, Chang HH, Jung SI, et al. Evidence for clonal dissemination of the serotype K1 Klebsiella pneumoniae strain causing invasive liver abscesses in Korea. J. Clin. Microbiol. 2008;46:4061–4063. doi: 10.1128/JCM.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung CP, Chang FY, Lee SC, Hu BS, Kuo BI, Liu CY, et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut. 2002;50:420–424. doi: 10.1136/gut.50.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn. Microbiol. Infect. Dis. 2008;62:1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Cryz SJ, Jr, Mortimer PM, Mansfield V, Germanier R. Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. J. Clin. Microbiol. 1986;23:687–690. doi: 10.1128/jcm.23.4.687-690.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JK, Chung DR, Wie SH, Yoo JH, Park SW. Risk factor analysis of invasive liver abscess caused by the K1 serotype Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2009;28:109–111. doi: 10.1007/s10096-008-0595-2. [DOI] [PubMed] [Google Scholar]
- 24.Lin JC, Siu LK, Fung CP, Tsou HH, Wang JJ, Chen CT, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J. Clin. Endocrinol. Metab. 2006;91:3084–3087. doi: 10.1210/jc.2005-2749. [DOI] [PubMed] [Google Scholar]
- 25.Chung DR, Lee H, Park MH, Jung SI, Chang HH, Kim YS, et al. Fecal carriage of serotype K1 Klebsiella pneumoniae ST23 strains closely related to liver abscess isolates in Koreans living in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:481–486. doi: 10.1007/s10096-011-1334-7. [DOI] [PubMed] [Google Scholar]
- 26.Struve C, Bojer M, Nielsen EM, Hansen DS, Krogfelt KA. Investigation of the putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J. Med. Microbiol. 2005;54(Pt. 11):1111–1113. doi: 10.1099/jmm.0.46165-0. [DOI] [PubMed] [Google Scholar]
- 27.Kang CI, Kim SH, Bang JW, Kim HB, Kim NJ, Kim EC, et al. Community-acquired versus nosocomial Klebsiella pneumoniae bacteremia: clinical features, treatment outcomes, and clinical implication of antimicrobial resistance. J. Korean Med. Sci. 2006;21:816–822. doi: 10.3346/jkms.2006.21.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NORM/NORM-VET 2012. 2012. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. Tromsø/Oslo.
- 29.Hope WW, Vrochides DV, Newcomb WL, Mayo-Smith WW, Iannitti DA. Optimal treatment of hepatic abscess. Am. Surg. 2008;74:178–182. [PubMed] [Google Scholar]
- 30.Yang CC, Yen CH, Ho MW, Wang JH. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J. Microbiol. Immunol. Infect. 2004;37:176–184. [PubMed] [Google Scholar]


