Figure 5. Phosphorylation of MPG is required for optimal its function in DNA repair.
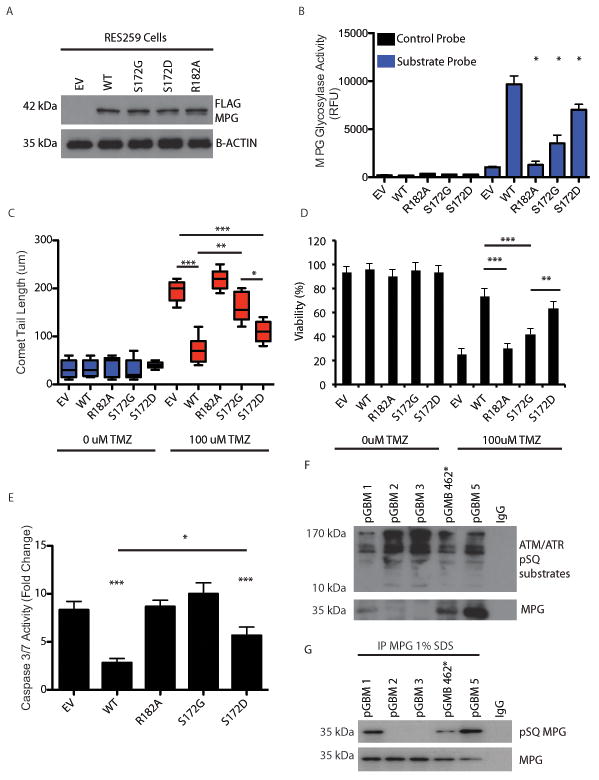
a. Immunoblot using anti-Flag antibody demonstrating robust expression of MPG flag tagged constructs in pediatric GBM cell line RES259. EV: empty vector, WT: wild type, S172G: MPG mutant in which the serine at residue 172 is replaced by glycine, S172D: MPG mutant in which the serine at residue 172 is replaced by aspartic acid, R182A: MPG mutant in which the arginine at residue 182 is replaced by alanine, B-actin: beta-actin.
b. Quantification of molecular beacon MPG activity assay after transfection of empty vector (EV), wild-type (WT) or mutant MPG constructs (R182A, S172G or S172D) in presence of TMZ (100uM).
c. Quantification (b) of comet tail assay (a) after transfection of empty vector (EV), wild-type (WT) or mutant MPG constructs (R182A or S172G) in presence (+) or absence (-) of TMZ (100uM).
d. Cell viability assay after transfection of wildtype or mutant MPG constructs in the presence or absence of TMZ at 48h.
e. Activated cleaved caspase 3/7 assay after transfection of wildtype or mutant MPG constructs in presence of TMZ (100uM).
f. Immunoprecipitation of ATM/ATR pSQ substrates in 5 frozen pGBM operative samples. Immuno precipitates were additionally probed for MPG and detected in 3/5 samples. IgG was used as a negative control.
g. Immunoprecipitation of MPG is 1% SDS followed by western blotting of pSQ from the 5 samples used in f to demonstrate that MPG is phosphorylated at the pSQ residue in clinical samples.
*P<0.05,**P<0.01***P<0.001. All experiments were performed in triplicate with mean and SEM reported where appropriate.
