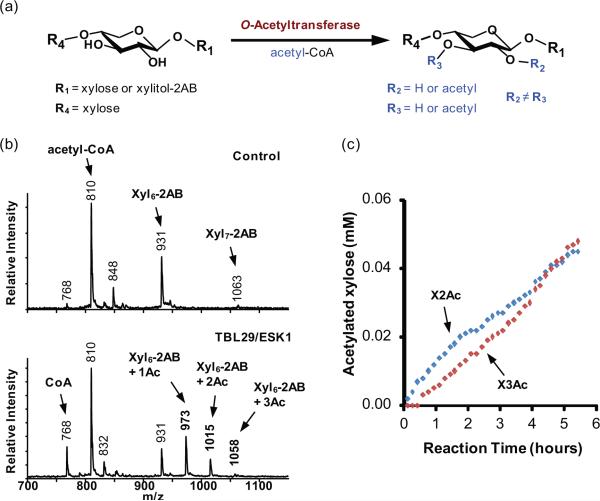
Figure 3. TBL29/ESK1 catalyzed acetylation of xylooligosaccharides using acetyl-CoA as the donor.
(a) In vitro O-acetyltransferase reaction scheme.
(b) MALDI-TOF MS of the products after incubating TBL29/ESK1 for 16 hours with Xyl6-2AB and acetyl-CoA. Each transfer of an O-acetyl (Ac) group increases the mass of the Xyl6-2AB acceptor by 42 Da to generate products corresponding to the annotated [M+H]+ ions.
(c) Amounts of acetylated xylosyl residues at O-2 (X2Ac) or O-3 (X3Ac) formed during the first 6 hours of the TBL29/ESK-catalyzed reaction. The product concentrations were determined by real time 1H NMR analysis.