Table 1.
Catalytic Decarboxylative Trifluoromethylation Improved by DMEDA and an Activation Procedurea
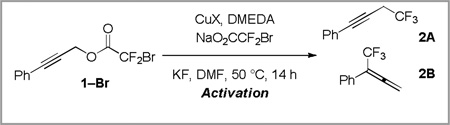 | ||||
|---|---|---|---|---|
| Entry | CuX (mol %) |
DMEDA (mol %) |
Activationb | % Yieldc (A : B)d |
| 1e | I (100) | 0 | – | 57 (2.6 : 1) |
| 2 | I (10) | 0 | – | 65 (3.3 : 1) |
| 3 | I (10) | 10 | – | 51 (3.6 : 1) |
| 4 | I (10) | 10 | √ | 75 (2.7 : 1) |
| 5 | TC (10) | 10 | √ | 52 (2.7 : 1) |
| 6f,g | TC (5) | 0 | – | <5 (N.D.) |
| 7f,h | TC (5) | 0 | – | 0 (–) |
Reactions were performed with 1-Br (0.20 mmol), and KF (0.40 mmol) in DMF (0.20 mL).
Activation involved heating CuI, DMEDA, NaO2CCF2Br, and KF in DMF for 10 min prior to injection of 1-Br.
Combined yield of 2A and 2B as determined by 19F NMR analysis, using α,α,α-trifluorotoluene as an internal standard.
Determined by 19F NMR spectroscopic analysis. ND = not determined.
DMF (0.60 mL).
KF (0.30 mmol), THF (1.2 mL), 20 h.
TMSCF3 (0.30 mmol) was added to the reaction.
75% of 1-Br remained, as determined by 19F NMR spectroscopic analysis.
