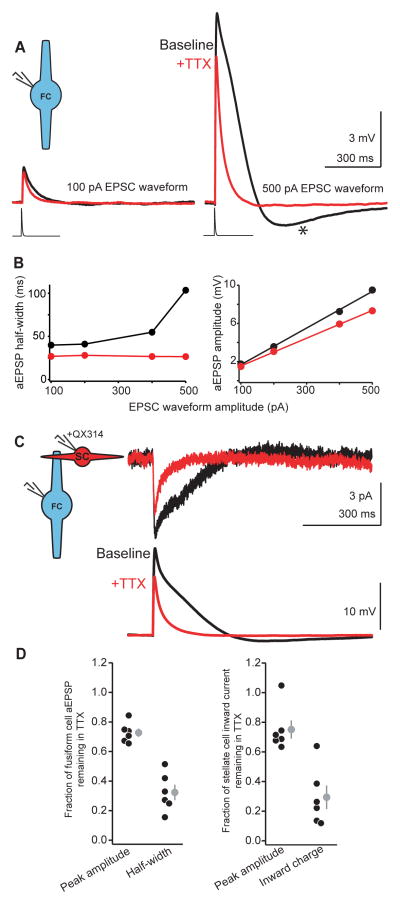
Figure 2.
Subthreshold activation of Na+ channels in fusiform cells shapes excitatory transmission in electrically-coupled stellate cells.
A) Example traces from a fusiform cell in current-clamp. An EPSC waveform is injected at two different amplitudes (100 and 500 pA) before and after bath application of TTX (black and red traces, respectively). The differential kinetics of the black traces highlights the role of Na+ channels in shaping the aEPSP half-width. TTX application reduced the aEPSP half-width and peak amplitude, showing that subthreshold depolarizations activate Na+ channels. Note the AHP following the aEPSP evoked with a 500 pA waveform. Resting voltages for 100 pA events: −70 mV (control), −71 mV (TTX); for 500 pA events: −72 mV (control), −71 mV (TTX).
B) Example input/output curves for the example cell shown in panel (A). Left panel: aEPSP half-widths as a function of EPSC waveform amplitude before and after TTX (black and red points, respectively). In n=7 cells, aEPSP half-widths evoked with the largest EPSC waveform amplitude (mean: 471±58 pA, range: 250–700 pA) were on average 2.4±0.4 times greater than aEPSP half-widths evoked with the smallest amplitude waveform (mean: 114±24 pA, range: 50–200 pA). This ratio was reduced to 1.0±0.1 in TTX (p=0.007, paired t-test), showing that Na+ channels powerfully control the kinetics of subthreshold events. Right panel: aEPSP peak amplitudes plotted as a function of EPSC command waveform in baseline and after TTX. Color scheme is the same as left panel. The dotted lines are linear fits to the data points to calculate the slope of the input/output function. On average, TTX reduced the slope of the aEPSP amplitude function to 77±3% of baseline (0.027±0.004 mV/pA in baseline to 0.020±0.003 mV/pA in TTX, n=7 cells, p=0.007, paired t-test).
C) Left: diagram of paired recording experiment. Na+ channels are blocked specifically in the stellate cell by the addition of the Na+ channel blocker QX314 to the stellate cell pipette internal solution. Right: Average traces showing a subthreshold aEPSP in a fusiform cell (lower panel) and the resulting inward current in the stellate cell (upper panel) before and after TTX (black and red traces, respectively). TTX reduced the amplitude and half-width of aEPSPs in the fusiform cell. TTX similarly reduced the amplitude and charge transfer of the postjunctional inward current in the stellate cell, showing that Na+ channels in fusiform cells are a significant source of excitation. Resting voltages for fusiform cell pre-TTX, −75 mV; post-TTX: −74 mV.
D) Summary data from 6 pairs similar to C. Left: TTX reduced the amplitude and half-width of fusiform cell aEPSPs to 73±3% and 32±5% of baseline (p=0.0002 and p<0.0001, respectively, one sample t-test). Right: TTX also significantly reduced the amplitude and inward charge transfer of postjunctional currents in stellate cells to 75±6% and 29±8% of baseline (p=0.0097 and p=0.0003, respectively, one sample t-test). Gray points are mean±SEM.