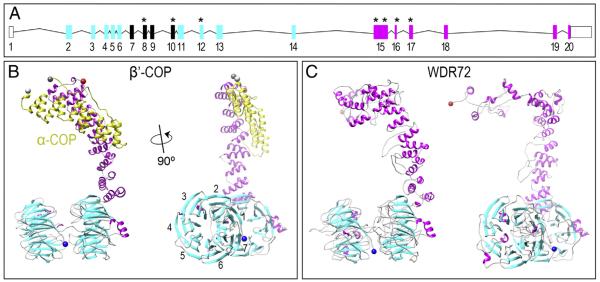
Fig. 2. WDR72 adopts the β-propeller and α-solenoid fold configuration characteristic of membrane-deformation complexes.
(A) The human Wdr72 gene with predicted WD40 domains (cyan boxes) and sequence homology divergence to all other human genes (magenta boxes). Reported mutations associated with Amelogenesis Imperfecta are denoted with an asterisk (*), showing our identified exon 8 mutation in between two clusters of WD40 repeat domains (ENST00000360509). Mutation positions based on CCDC 10151.1; numbered box, Wdr72 exon; line, intron; empty box, untranslated region (UTR). (B) Monomeric structure of β′-COP protein interacting with the C-terminus of α-COP (yellow), another subunit of the crystallized Saccharomyces cerevisiae COPI triskelion vesicle coat complex (PDB identifier: 3mkq). The side view of β′-COP (right) depicts what is referred to as a βeta-propeller structure (cyan) and an α-solenoid tail (magenta) unique to proteins that interact with curved lipid bilayers. This β′-COP structure was used as a template for model building, as it showed the greatest similarity to WDR72 compared to any other solved protein structures. Numbers represent blades #1–7 that comprise a single β-propeller. (C) Constructed model of WDR72 showing two β-propeller domains and a curved α-solenoid tail. The side view of full-length WDR72 (right) depicts two β-propellers, each with 7 blades (cyan), and a series of α-helices (magenta) forming an α-solenoid tail. Blue sphere, N-terminus; red sphere, C-terminus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)