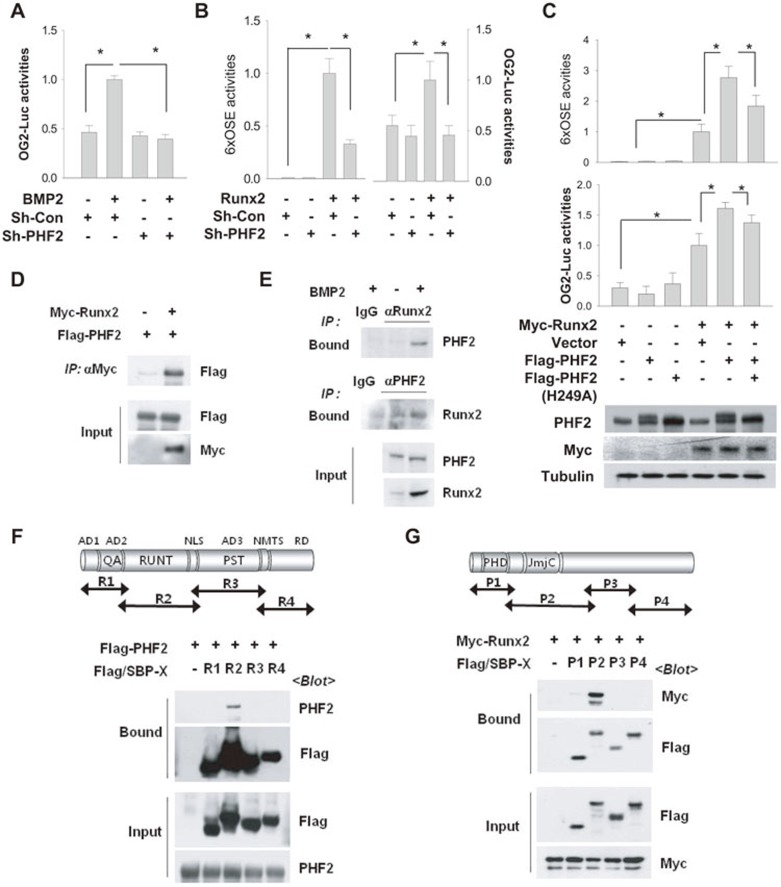
Figure 5.
PHF2 interacted with Runx2 to activate Runx2. (A) C2C12 stable cell lines expressing control (Sh-Con) or PHF2-targeting (Sh-PHF2) shRNA were transfected with OG2 promoter-luciferase reporter and β-gal plasmids and treated with 50 ng/ml of BMP2 for 24 h. Bars represent the mean ± SD (n = 4) for relative luciferase activity. * denotes P < 0.05 for the indicated groups. (B) C2C12 stable cell lines were co-transfected with Myc-Runx2, luciferase reporter, and β-gal plasmids, and incubated for 48 h. Bars represent the mean ± SD (n = 4). (C) C2C12 cells were co-transfected with Myc-Runx2, Flag vector, Flag-PHF2, Flag-PHF2 (H249A), 6×OSE-luciferase, OG2 promoter-luciferase, and β-gal plasmids in the indicated combinations. Results (luciferase/β-gal) are presented as the relative values to the BMP2/Sh-Con group. Bars represent the mean ± SD (n = 4). (D) HEK293T cells were co-transfected with Myc-Runx2 and Flag-PHF2 plasmids. Cell extracts were immunoprecipitated with an anti-Myc antibody, and coprecipitated Flag-PHF2 was identified using anti-Flag. (E) After C2C12 cells were stimulated with BMP2 for 24 h, endogenous Runx2 or PHF2 was precipitated with anti-Runx2 or anti-PHF2, and coprecipitated proteins were immunoblotted. (F) The top panel shows a schematic diagram of Runx2. HEK293T cells were co-transfected with Flag-PHF2 and one of four plasmids for Flag/SBP-Runx2 fragments. Runx2 fragments were pulled down using streptavidin beads, and the precipitated proteins were immunoblotted. AD1-3, transactivation domain 1-3; NLS, nucleus localization signal; NMTS, nuclear matrix-targeting signal; RD, repression domain; QA, polyglutamine and polyalanine domain; PST, proline/serine/threonine-rich domain. (G) The top panel shows a schematic diagram of PHF2. HEK293T cells, which had been co-transfected with Myc-Runx2 and one of four F/S-PHF2 fragments, were subjected to streptavidin pull-down, and the precipitated proteins were immunoblotted.