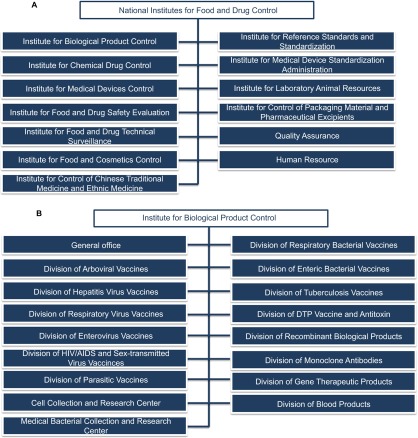
Figure 1.
Organization structure. (A) The Divisions of NIFDC and (B) the Divisions of Institute for Biological Product Control. The Division of Recombinant Biological Products is in charge of establishing quality standards and evaluation methods for biotechnology drugs. The Division of Respiratory Virus Vaccines is responsible for quality control and for evaluating the pandemic influenza vaccine. The Division of Hepatitis Virus Vaccines is in charge of the quality control and evaluation of HFMD vaccines.