SYNOPSIS
Childhood asthma and obesity have reached epidemic proportions worldwide, and the latter is also contributing to increasing rates of related metabolic disorders like diabetes. Yet, the relationship between asthma, obesity, and abnormal metabolism is not well understood, nor has it been adequately explored in children. This article discusses the concept of “metabolic asthma” and the recent hypothesis that early derangement in lipid and glucose metabolism is independently associated to increased risk for asthma.
Keywords: Body Mass Index, Fetal Programming, Lung Development, Metabolic Syndrome
Childhood obesity has reached epidemic proportions worldwide, prompting first lady Michelle Obama to launch the “Let’s Move!” campaign against childhood obesity in February 2010 [1]. Overweight is currently defined as a body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) at the 85th to less than the 95th percentile for age, whereas obesity is defined as a BMI at or above the 95th percentile (Figure 1). Data from the Centers for Disease Control and Prevention (CDC) indicate that today, nearly one in three children in America are overweight or obese and that the majority of the United States has already passed the 30% mark.
Figure 1. Body Mass Index.

The Centers for Disease Control and Prevention (CDC) define overweight as a body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) at the 85th to less than the 95th percentile for age, whereas obesity is defined as a BMI at or above the 95th percentile. Today, nearly one in three children in America are overweight or obese and the majority of the United States has already passed the 30% mark.
What is especially concerning is the rate at which this problem is growing: over the past 3 decades, childhood obesity rates in America have tripled. Furthermore, numbers are even higher in African-Americans and Hispanics, with nearly 40% of children overweight or obese. If this trend does not change, it is estimated that one third of all children born in 2000 or later will suffer at some point in their lives from co-morbidities typically linked to excessive weight. In particular, the prevalence of metabolic syndrome has increased significantly and today more than 2 million children in the United States have this condition defined by systemic hypertension, atherogenic dyslipidemia and glucose intolerance.
Obesity-Asthma Link
A similar epidemiologic pattern has been observed for chronic respiratory diseases, particularly asthma. Four million children under 14 years of age have been diagnosed with asthma in the United States [2] and the current global estimates of asthma prevalence range from less than 5% to more than 25% [3]. The parallel rise in obesity and asthma rates among children has led many to postulate a relationship between these pathologies [4–7], although it remains controversial whether such relationship is causal or confounded by other factor(s).
Previous studies of the association between asthma and obesity have focused on 3 hypothetical mechanisms. The most simplistic theory is centered on the role of specific nutrients like anti-oxidants and saturated fat [4], which may lead to oxidative lung damage or decrease lung defenses against the attacks from biological or chemical agents. The recent emphasis on the potential role of vitamin D deficiency in the pathophysiology of a number of chronic diseases driven by immunologic or autoimmune mechanisms – including asthma – has given new life to this idea, but at this time the conclusions from interventional trials with high-dose vitamin D supplementation remain controversial.
A second theory is centered on the mechanical effects of abdominal fat on respiratory system resistance and compliance [5, 6]. Obesity reduces total lung capacity (TLC), particularly by decreasing the expiratory reserve volume (ERV) and consequently the functional residual capacity (FRC). This leads to the rapid, shallow breathing pattern that occurs close to closing volume in obese subjects. Perhaps more importantly, breathing at low TLC is associated with reduced peripheral airway diameter, and this in turns alters bronchial smooth muscle structure and function leading to airway hyperresponsiveness.
The third theory, which is also the most recent and probably most widely accepted, is based on the inflammatory mechanisms implicated in both conditions [5, 8]. In obesity, visceral adiposity is associated with increased expression of multiple soluble mediators that amplify and propagate inflammation both locally and systemically. This involves the recruitment of inflammatory cells by chemokines such as monocyte chemoattractant protein-1 (MCP-1), as well as the direct synthesis of predominantly pro-inflammatory cytokines and chemokines such as leptin, interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), transforming growth factor β1 (TGF-β1), and eotaxin. The resulting perturbation of the balance between Th1 and Th2 immunomodulatory pathways – favoring the latter - has been hypothesized to be one of the mechanisms by which obesity might increase asthma risk or modify asthma phenotype.
Diabetes-Asthma Link
In addition to the hypothetical relationship between obesity and asthma, there is strong evidence that obesity is associated with the development of insulin resistance and type II diabetes [9]. In turn, diabetes and insulin resistance are associated with diminished lung function [10–12], and some studies have also found a relationship between insulin resistance and lung function among non-diabetics, even after controlling for BMI (Figure 2).
Figure 2. Obesity-Diabetes-Asthma Link.
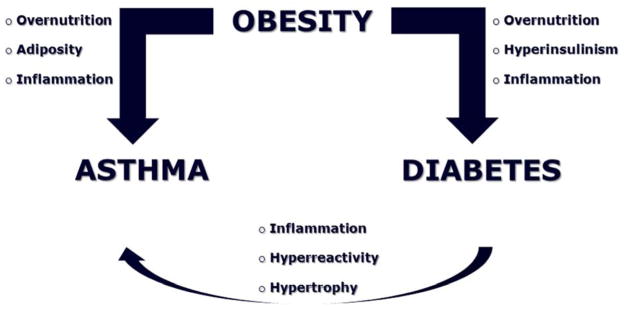
In addition to the hypothetical relationship between obesity and asthma, there is strong evidence that obesity is associated with the development of insulin resistance and type II diabetes. In turn, diabetes and insulin resistance are associated with diminished lung function, and some studies have also found a relationship between insulin resistance and reduced lung function among non-diabetics, even after controlling for BMI.
Therefore, increasing rates of pediatric asthma could result directly from peripheral tissue insulin resistance and compensatory hyperinsulinemia, which interfere with the anti-inflammatory effects of insulin while increasing bronchial reactivity through inhibition of pre-synaptic M2 muscarinic receptors [13]. In addition, intracellular serine/threonine kinases like c-Jun NH(2)-terminal protein kinases (JNKs) are activated by toll-like receptor signaling in the context of innate immuno-inflammatory responses, and also inhibit insulin signaling [14]. Because of the close interdependence between inflammatory and metabolic pathways, pharmacologic ligands of the peroxisome proliferator-activated family of nuclear receptor proteins (PPARs) widely used to treat hyperlipidemia and diabetes may also treat the airway inflammation and hyperreactivity characteristic of asthma.
Metabolic Origins of Asthma
Obesity has generally been thought to be the central hub from which co-morbidities like asthma, cardiovascular disease, and metabolic syndrome originate. Because of this bias, most of the studies designed to examine the interactions between childhood asthma and obesity were based on select cohorts of obese children. A few years ago, we reasoned that new and important information could result from studies looking at larger, more heterogeneous samples of children stratified by body mass.
A unique opportunity to fill this knowledge gap became available through collaboration with the “Coronary Artery Risk Detection In Appalachian Communities” (CARDIAC) Project, a federally- and state-funded, community-based cardiovascular risk detection program offered to all children enrolled in kindergarten, 2nd, and 5th grade classrooms in the state of West Virginia [15, 16]. Every year, this project screens tens of thousands of children for BMI. The screening staff also explores the base of the child’s neck and axilla for evidence of Acanthosis Nigricans (Figure 3) – a hyperpigmented skin rash associated with insulin resistance and hyperinsulinemia in children [17] - and a blood sample is drawn from 5th grade students only to obtain a fasting lipid profile. Prior to screening day, all participating parents provide demographic and family history information by completing a questionnaire.
Figure 3. Acanthosis Nigricans (AN).
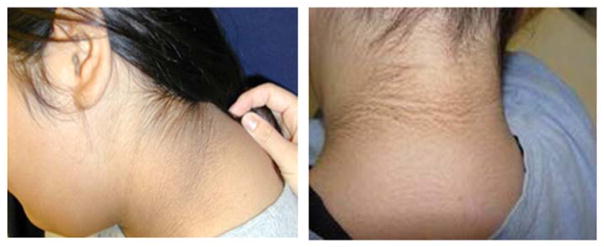
This hyperpigmented skin rash is usually found at the base of the neck and axilla and is highly predictive of insulin resistance in adults. Although less documented in children, recent data indicate that more than 60% of children with AN also have a homeostatic model assessment index (HOMA) ≥3. HOMA is the current gold standard of insulin resistance and beta-cell function based on fasting plasma glucose and insulin levels.
Analysis of almost 18,000 children enrolled during the academic year 2007–2008 provided the first conclusive evidence of the relationship between asthma and body mass in a large population of children across the entire range of weight percentile categories [18]. This analysis confirmed that asthma prevalence increases with BMI, but only when BMI reaches the obese and morbidly obese range, whereas there is no difference between overweight vs. normal weight children (Figure 4). This observation suggests the existence of a threshold beyond which the metabolic derangement starts affecting airway function. Importantly, our study showed similar results in both genders, whereas a previous study in a much smaller sample had suggested that body mass may affect asthma prevalence only in girls.
Figure 4. Asthma prevalence based on weight category.
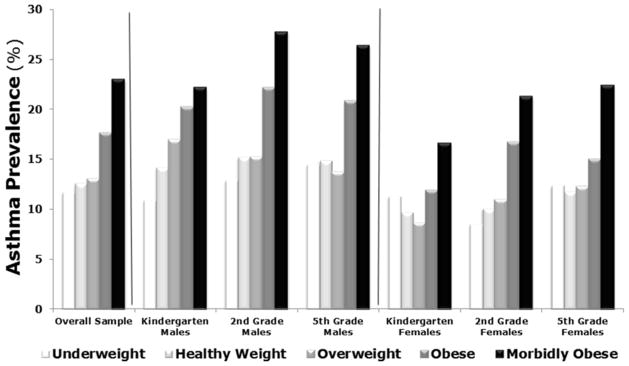
As a general trend, asthma prevalence increases as children’s BMI percentile increases. Asthma prevalence in obese and morbidly obese children is significantly higher than in children with healthy BMI, whereas simple overweight status does not increase this risk. Asthma patterns in obese children are similar for males and females and across ages.
Adapted from L. Cottrell, W. A. Neal, C. Ice, M. K. Perez, and G. Piedimonte “Metabolic Abnormalities in Children with Asthma”, American Journal of Respiratory and Critical Care Medicine, 183:441–448, 2011; with permission.
The most important conclusion of our studies is that asthma is directly associated with elevated serum triglyceride levels and insulin resistance regardless of BMI. This contributes to the mounting concerns for a potentially large population of children - sometime referred to as being “thin-fat” - who are metabolically obese despite having a deceivingly normal weight. These children risk to be overlooked because they have a healthy appearance based on weight and adiposity; yet their metabolism is already abnormal and is predisposing them to the same cardiovascular and respiratory comorbidities typical of their overweight peers.
Another indirect implication of our data is that the widely reported association asthma-obesity may have been confounded by the coexistence of hypertriglyceridemia and insulin resistance. Indeed, children with physician-diagnosed asthma tend to have higher serum triglyceride levels and higher rates of insulin resistance regardless of the children’s body mass. Thus, dyslipidemia and hyperinsulinemia, known silent precursors to cardiovascular disease and diabetes, may also be associated to the development of asthma and confound its epidemiologic link to obesity.
Our findings imply a strong and direct influence of metabolic pathways on the immune mechanisms, both innate and adaptive, involved in the pathogenesis of asthma in children, and suggest that strict monitoring and dietary/pharmacologic control of triglyceride and glucose levels starting in the first years of life may have an important role in the management of chronic asthma in children.
Prenatal influences
As metabolic events have been implicated in the pathophysiology of airway inflammation and hyperreactivity, it is conceivable that early-life abnormalities in lipid and/or glucose metabolism contribute to the pathogenesis of asthma in childhood. Indeed, there is mounting evidence that the nine months spent in our mother’s uterus and the first months after our birth shape the rest of our lives define our medical destiny.
This concept may be true also for the most common respiratory pathologies, like asthma and chronic obstructive pulmonary disease (COPD). Preliminary data from our lab suggest that imbalanced diet in pregnancy interferes with lung development and innervation leading to postnatal airway hyperreactivity independently from post-natal diet. Furthermore, prenatal diet can also affect the development of innate and adaptive immune protection, thereby making the infant more susceptible to early-life infections like respiratory syncytial virus (RSV) and human rhinovirus (HRV) that predispose to recurrent wheezing and asthma in childhood.
The general idea we have been working under for decades is that nothing bad happens to the lungs until the baby is born — except in rare cases of congenital malformations or if gestation is cut short by a preterm delivery – and that the earliest manifestations of lung disease stem from noxious agents attacking the newborn lungs after birth. But if the maternal diet affects lung development, our understanding of the pathogenesis of respiratory diseases would be completely changed. It would turn back the clock of respiratory developmental diseases by months and we would need to start thinking about lung development and pathology during pregnancy rather than at birth.
This could create a paradigm shift by extending our focus on prevention from the first few years after birth to also include the last few months before birth. The new paradigm is in line with the emerging evidence that many (or most) chronic inflammatory, degenerative, and even neoplastic diseases plaguing adults have their origins from often-subtle events occurring during fetal life. The “fetal programming hypothesis” was originally formulated by Dr. David Barker more than two decades ago to explain the extensively reproduced and confirmed epidemiologic evidence that low birth weight predisposes to cardiovascular disease in late adulthood.
Dr. Barker recent death, leaves us with the legacy of this initially controversial, but now widely accepted, idea that common chronic illnesses such as cancer, cardiovascular disease and diabetes result not always from bad genes and an unhealthy adult lifestyle, but from poor intrauterine and early postnatal health. In one of his last public speeches, he argued: “The next generation does not have to suffer from heart disease or osteoporosis. These diseases are not mandated by the human genome. They barely existed 100 years ago. They are unnecessary diseases. We could prevent them had we the will to do so.”
We believe the same concepts can be extended to chronic obstructive airway diseases like asthma, which is the final product of complex interactions between genetic and environmental variables (Figure 5). Prenatal events like the intrauterine exposure to imbalanced maternal nutrition, infections, or pollutants will cause a shift in the trajectory of structural and functional airway development toward a hyperreactive phenotype. The same intrauterine exposures can affect gene expression via epigenetic modifications like DNA methylation, histone acetylation, and by altering the relative expression of regulatory micro-RNAs.
Figure 5. Fetal programming of asthmatic airways.
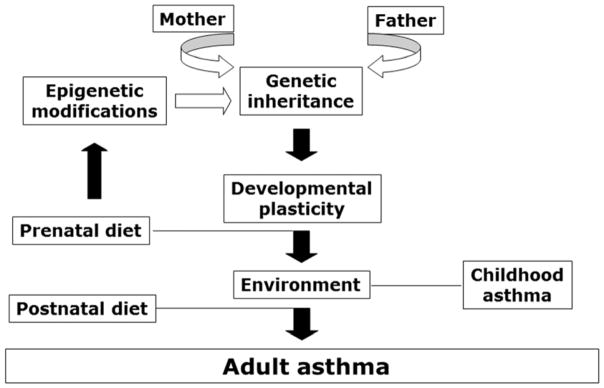
What makes our lungs prone to develop chronic disease? Of course, genetic traits inherited from our parents are important role. But also the quantity and quality of food our mother eats, the pollution in the air she breathes, the infections she suffers during gestation will play a critical role throughout our life, perhaps even more important than genetics. In particular, prenatal events like the intrauterine exposure to maternal unbalanced diet may cause a shift in the trajectory of structural and functional airway development toward a hyperreactive phenotype. The resulting neonatal phenotype may predispose the child to aberrant responses to common respiratory infections and airborne irritants, thereby increasing the risk of obstructive lung disease later in life. Postnatal events, such as exposure to indoor and outdoor pollutants and allergens, can further shift the equilibrium of the adult phenotype by exacerbating airway inflammation and hyperreactivity.
The resulting neonatal phenotype will predispose the child to aberrant responses to common respiratory infections and airborne irritants, thereby increasing the risk of obstructive lung disease later in life. Postnatal events, such as exposure to indoor and outdoor pollutants and allergens, can further shift the equilibrium of the adult phenotype by exacerbating airway inflammation and hyperreactivity. The continuous range of possible developmental trajectories and multiple sequential events acting during development will define the severity and duration of disease.
Dr. Barker believed that public health medicine was failing and that its cornerstone should be the protection of the nutrition of young women. Indeed, chronic disease like obesity, diabetes and asthma are becoming epidemic and their management in adulthood is escalating the costs of healthcare to proportions unsustainable for any world economy. If we want to prevail in the war against chronic airway diseases that so far have eluded any therapeutic strategy, it is essential to recognize that the months spent in our mothers’ womb may be the most consequential of our lives, and identify the intrauterine and early life events shaping the development of the respiratory system to prevent or redirect dysfunctional phenotypes before they result in actual disease. Securing a balanced diet to all pregnant women and their newborns may become one day one of the first and most important steps in this direction.
KEY POINTS.
Regardless of their body mass index percentile, children diagnosed with asthma are more likely than non-asthmatics to have higher triglyceride and insulin blood levels.
Dyslipidemia and hyperinsulinemia, known silent precursors to cardiovascular disease, are also associated to the development of asthma and confound its epidemiologic link to obesity.
Diet and physical exercise may influence the development and persistence of immune mechanisms - both innate and adaptive - involved in the pathogenesis of asthma in children.
Prenatal events like the intrauterine exposure to imbalanced maternal nutrition may cause a shift in the trajectory of structural and functional airway development toward a hyperreactive phenotype.
Monitoring and dietary/pharmacologic control of triglyceride and glucose metabolism during pregnancy and in the first years of life may become an important component of the prevention and management of asthma.
Acknowledgments
The Authors are very grateful to Dr. William Neil and his CARDIAC Project without whom the research on the link between asthma, diabetes and obesity would not have been possible. We also thank the National Heart, Lung and Blood Institute of the National Institute of Health for the generous support of our research over the past 15 years.
Footnotes
The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wojcicki JM, Heyman MB. Let’s Move - Childhood Obesity Prevention from Pregnancy and Infancy Onward. N Engl J Med. 2010;362:1457–1459. doi: 10.1056/NEJMp1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. Geneva: World Health Organization; 2007. [Google Scholar]
- 3.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 4.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 5.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinn S. Obesity and asthma: evidence for and against a causal relation. J Asthma. 2003;40:1–16. doi: 10.1081/jas-120017202. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez MA, Winkleby MA, Ahn D, Sundquist J, Kraemer HC. Identification of population subgroups of children and adolescents with high asthma prevalence: findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2002;156:269–275. doi: 10.1001/archpedi.156.3.269. [DOI] [PubMed] [Google Scholar]
- 8.Weiss ST, Shore S. Obesity and asthma: directions for research. Am J Respir Crit Care Med. 2004;169:963–968. doi: 10.1164/rccm.200303-403WS. [DOI] [PubMed] [Google Scholar]
- 9.Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. 2003;88:1417–1427. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- 10.Engstrom G, Hedblad B, Nilsson P, Wollmer P, Berglund G, Janzon L. Lung function, insulin resistance and incidence of cardiovascular disease: a longitudinal cohort study. J Intern Med. 2003;253:574–581. doi: 10.1046/j.1365-2796.2003.01138.x. [DOI] [PubMed] [Google Scholar]
- 11.Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and Type 2 diabetes: findings from the British Women’s Heart and Health Study. Diabetologia. 2004;47:195–203. doi: 10.1007/s00125-003-1310-6. [DOI] [PubMed] [Google Scholar]
- 12.McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2005;161:546–556. doi: 10.1093/aje/kwi076. [DOI] [PubMed] [Google Scholar]
- 13.Al-Shawwa BA, Al-Huniti NH, DeMattia L, Gershan W. Asthma and insulin resistance in morbidly obese children and adolescents. J Asthma. 2007;44:469–473. doi: 10.1080/02770900701423597. [DOI] [PubMed] [Google Scholar]
- 14.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demerath E, Muratova V, Spangler E, Li J, Minor VE, Neal WA. School-based obesity screening in rural Appalachia. Prev Med. 2003;37:553–560. doi: 10.1016/j.ypmed.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Neal WA, Demerath E, Gonzales E, Spangler E, Minor VE, Stollings R, Islam S. Coronary Artery Risk Detection in Appalachian Communities (CARDIAC): preliminary findings. WV Med J. 2001;97:102–105. [PubMed] [Google Scholar]
- 17.Hud JA, Jr, Cohen JB, Wagner JM, Cruz PD., Jr Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941–944. [PubMed] [Google Scholar]
- 18.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2011;183:441–448. doi: 10.1164/rccm.201004-0603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


