Fig. 3. GIPC1/synectin is required for CendR endocytosis downstream of NRP1 binding.
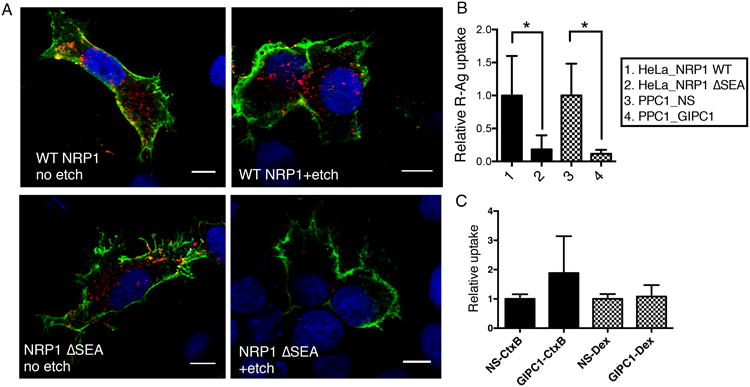
(A) NRP1-GIPC1/synectin interaction is important for the internalization of CendR peptides into cells. HeLa cells were transfected with plasmids expressing wild-type (WT) NRP1, or NRP1 ΔSEA mutant as described in Methods. R-Ag (red) was added 48 h later and incubated with the cells for 2 h at 37°C. The cells were then etched or washed twice with PBS without etching, fixed, and stained for cell surface NRP1 (green). Nuclei were stained with Hoechst 33342 (blue). Three independent experiments were performed and representative images are shown. Scale bar, 10 μm.
(B) Quantification of R-Ag uptake in (A). The fluorescence intensity of R-Ag per NRP1-positive HeLa cells in (A) were quantified and normalized to the average of WT NRP1-expressing samples as relative uptake (y-axis). As a comparison, PPC1 cells were treated with NS or GIPC1/synectin siRNA, and then incubated with R-Ag for 1 h before etching as described in Methods. The R-Ag signal per cell was quantified and normalized to the average of NS treated samples as relative uptake (y-axis). *P<0.05 (Student's t-test).
(C) GIPC1/synectin is not required for cellular uptake of non-CendR endocytic probes. After treatment with negative control siRNA (NS) or siRNA targeting GIPC1/synectin, the uptake of CtxB or Dex by PPC1 cells was measured as described in Methods. The fluorescence intensity of each probe per cell was normalized to the average of the corresponding negative controls (NS siRNA treated) as relative uptake (y-axis).
Error bars indicate SEM (3-6 replicates).
