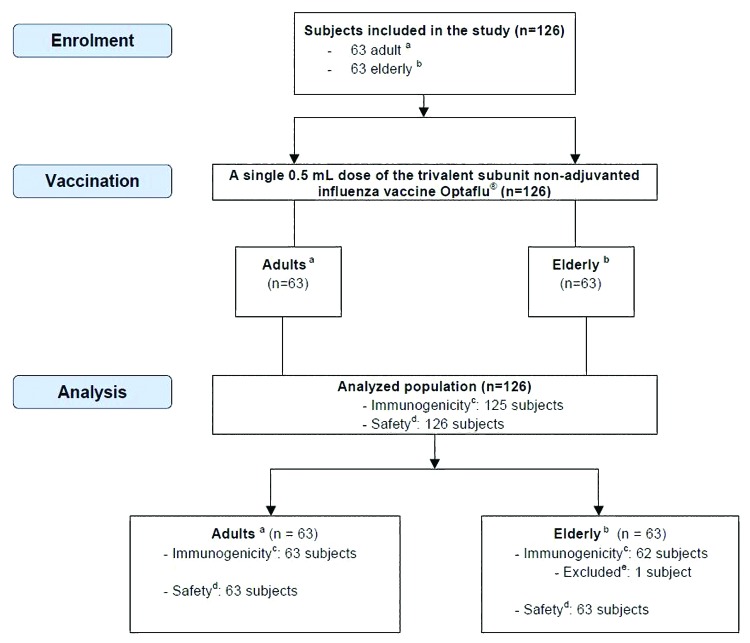
Figure 1. Inclusion and exclusion of subjects. aAdults (≥18–≤60 y); belderly (≥61 y); cimmunogenicity was analyzed as per protocol using HI and SRH assays according to CHMP criteria; dsafety assessment was conducted by collection of any solicited local and systemic reactions and collection of adverse events (adverse events were defined as solicited reactions persisting after day 4 or other than solicited local and systemic reactions reported during the study period); eone subject was not available for visit 3 and was therefore excluded from immunogenicity analysis

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.