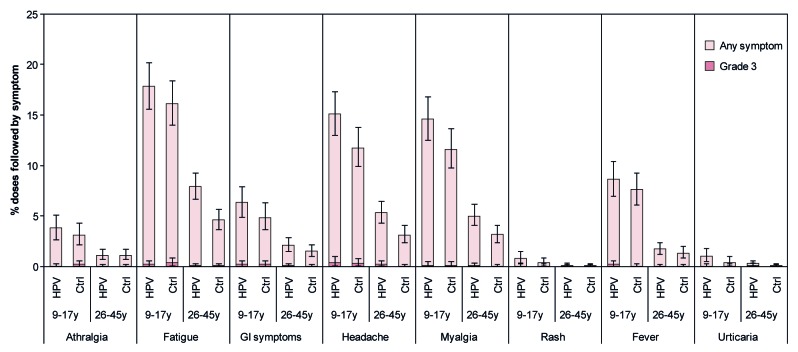
Figure 6. Solicited general symptoms reported during the 7-d period following any vaccine dose. Data are shown for the total vaccinated cohort. Bars represent the percentage of doses followed by the specified symptom at least once in the 7-d period after any vaccine dose with exact 95% confidence interval. Ctrl, control; HPV, HPV vaccine; GI, gastrointestinal. The control was Al(OH)3 for females aged 9–17 y and hepatitis B vaccine for females aged 26–45 y. Fever defined as oral/axillary temperature >37.0 °C; grade 3 fever defined as oral/axillary temperature >39.0 °C. For all other symptoms, grade 3 defined as symptom that prevents normal activity. Data for females aged 9–17 y are from Study HPV-058. Data for females aged 26–45 y are from Study HPV-069.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.