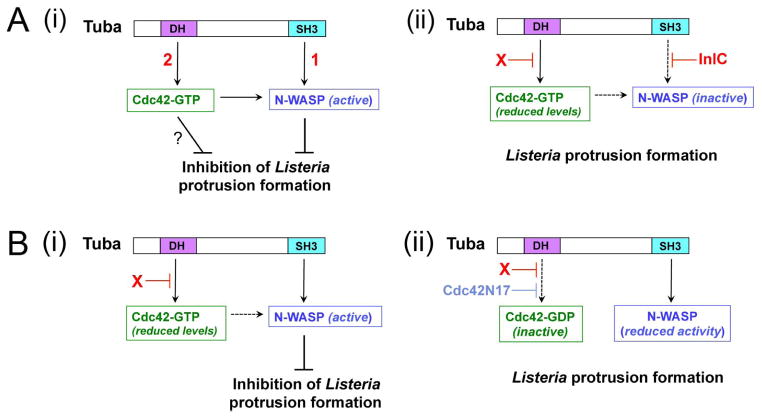
Figure 7. Mechanisms by which host Tuba and Cdc42 control Listeria protrusion formation.
(A). Model for how Listeria factors relieve Tuba- and Cdc42-mediated inhibition of Listeria spreading. A key feature of this model is that both Tuba and Cdc42 control N-WASP activity in an additive fashion. (i). In the absence of intervention by InlC or other bacterial proteins, Listeria protrusion formation is restrained by Tuba-mediated activation of N-WASP [1] and also by Cdc42-GTP [2]. Activated Cdc42 impairs protrusions by contributing to activation of N-WASP and also possibly through an unknown N-WASP-independent mechanism (?). (ii). InlC prevents binding of N-WASP to Tuba (Rajabian et al., 2009; Polle et al., 2013). An unknown bacterial factor ‘X’ impairs activation of Cdc42. Experiments involving depletion of Tuba in infected cells suggest that ‘X’ might attenuate Cdc42 activity by acting through Tuba. (B). Model explaining the spreading defect of ΔinlC mutant Listeria and the suppression of this defect by Cdc42N17. (i). In Caco-2 BBE1 cells infected with the ΔinlC mutant, N-WASP binds the Tuba SH3 domain. In the presence of reduced levels of Cdc42-GTP, interaction of Tuba with N-WASP produces sufficient N-WASP activity to impair bacterial protrusion formation. (ii). The Cdc42N17 mutant allele decreases Cdc42-GTP to such low levels that binding of the Tuba SH3 domain fails to activate N-WASP sufficiently to inhibit protrusions by the ΔinlC mutant.