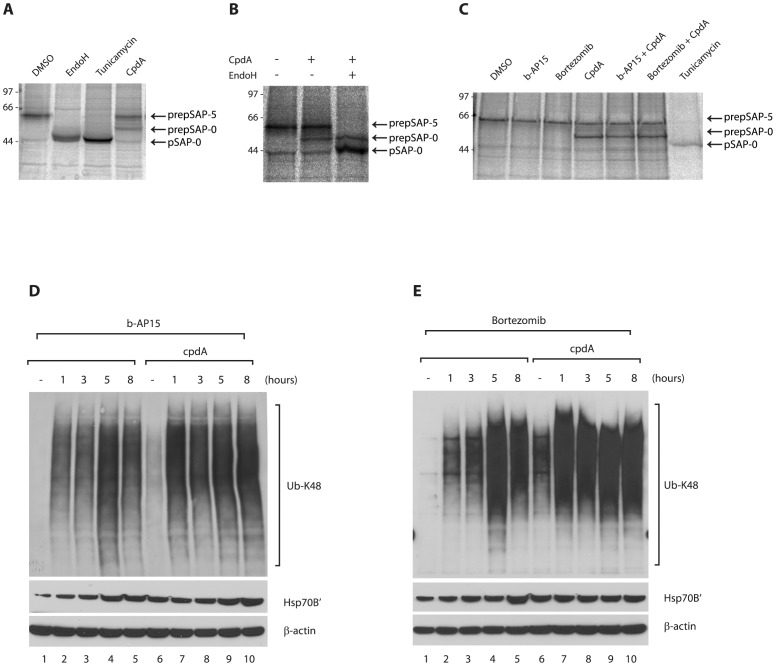
Figure 2. CpdA inhibits co-translational translocation of endogenous prosaposin into the ER.
A. and B. HCT116 cells were treated for 1 h with DMSO, tunicamycin (10 µg/ml) or CAM741 (10 µM) before pulse-labelling for 10 min with [35S] Met/Cys. Endogenous prosaposin (pSAP) was recovered by immunoprecipitation and newly synthesised pSAP species were visualised by phosphorimaging. In DMSO-treated cells, pSAP was fully glycosylated (pSAP-5), whereas Endo H digestion or tunicamycin treatment yielded non-glycosylated pSAP (pSAP-0). Inhibition of protein translocation into the ER by CAM741 resulted in the appearance of a pSAP species that migrated more slowly than the non-glycosylated protein and was Endo H-resistant. This species may represent signal sequence-containing preprosaposin (prepSAP-0) that has failed to translocate across the ER membrane. C. Distinct forms of endogenous pSAP in HCT116 cells treated with DMSO, b-AP15 (1 µM), bortezomib (20 nM), cpdA (10 µM) or tunicamycin (10 µg/ml) were recovered by immunoprecipitation and visualised by phosphorimaging. Treatment with cpdA specifically inhibits the co-translational translocation of pSAP into the ER as judged by the appearance of prepSAP-0 species.