Table 1. Calculated and experimental ZM241385 relative binding free energies for A2AAR mutants.
| Mutant |
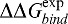 a
a
|
Radioligand |
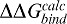
|
| V843.32A | NBb (>1.4) [30] | [3H]XAC | 3.7±0.4 |
| T883.36A | 0.9±0.5 [27] | [3H]XAC | 0.8±0.5 |
| Q893.37A | −0.6±0.1 [27] | [3H]XAC | −0.8±0.4 |
| S903.38A | −0.2±0.1 [27] | [3H]XAC | 0.2±0.4c |
| S913.39A | 0.4±0.1 [27] | [3H]XAC | −0.1±1.0c |
| F1685.29A | NBb (>1.4) [25] | [3H]ZM241385 | 2.2±0.4 |
| E1695.30A | NBb (>1.5) [28] | [3H]XAC | 2.7±1.5 |
| M1775.38A | 1.2±0.2 [25] | [3H]ZM241385 | 1.2±0.8 |
| L2496.51A | NBb (>1.4) [25] | [3H]ZM241385 | 5.7±0.7 |
| H2506.52A | NBb (>2.3) [29] | [3H]XAC | 2.8±0.7 |
| N2536.55A | NBb (>2.3) [29] | [3H]XAC | 4.5±0.5 |
| I2747.39A | NBb (>1.4) [29] | [3H]XAC | 5.4±1.0 |
| S2777.42A | −0.2±0.2 [29](XAC)d−0.1±0.2 (CGS15943) | [3H]XAC | 0.3±0.3 |
| H2787.43A | NB (>2.3) [29] | [3H]XAC | 3.5±1.5 |
Experimental relative binding free energies (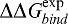 ) calculated from K
i values as
) calculated from K
i values as 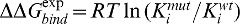 .
.
NB = non-detectable radioligand binding. The value corresponding to the experimental detection threshold is indicated within parentheses.
A Simulation sphere of 34 Å radius was used, since the mutated position is outside the boundaries of the default 25 Å sphere.
Experimental data is only available for the antagonists XAC and CGS15943.
