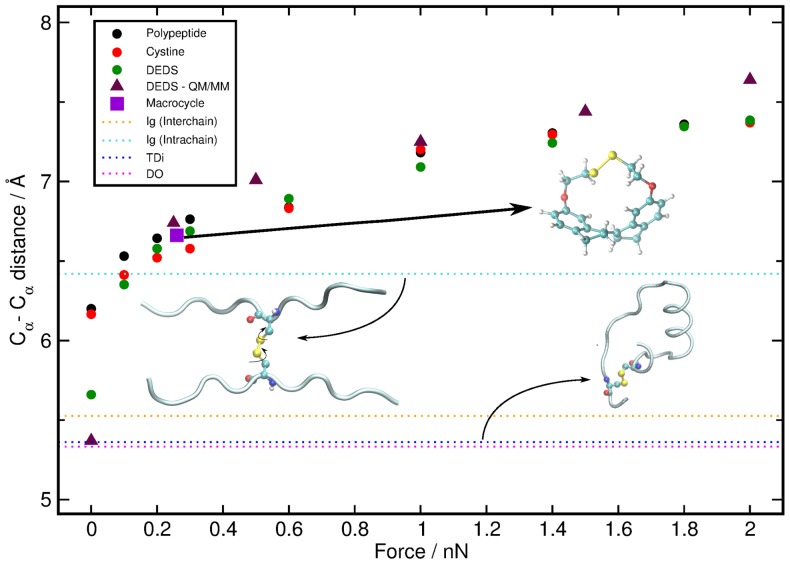
Figure 4. Stress–strain relation of disulfide bonds parameterized by the response of the Cα–Cα distance to tensile force.
Dependence of the computed average distance between the Cα–atoms as a function of 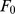 for the polypeptide model (black circles), cystine (red circles), and DEDS (green circles) depicted in Fig. 1 and obtained from force field equilibrium (at zero force) and force clamp MD (for
for the polypeptide model (black circles), cystine (red circles), and DEDS (green circles) depicted in Fig. 1 and obtained from force field equilibrium (at zero force) and force clamp MD (for 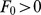 nN) simulations. Computational reference data for DEDS obtained from QM/MM simulations are shown by brown triangles and the experimental reference based on the strained macrocycle [27] (see text) is marked by a violet square. The horizontal blue, pink and orange dotted lines are the average Cα–Cα distances of disulfide bonds in TDi, DO and interchain Ig proteins, respectively, whereas the cyan dotted line corresponds to intrachain Ig proteins; these averages have been computed using the identical data sets as those that underly Fig. 3, see caption.
nN) simulations. Computational reference data for DEDS obtained from QM/MM simulations are shown by brown triangles and the experimental reference based on the strained macrocycle [27] (see text) is marked by a violet square. The horizontal blue, pink and orange dotted lines are the average Cα–Cα distances of disulfide bonds in TDi, DO and interchain Ig proteins, respectively, whereas the cyan dotted line corresponds to intrachain Ig proteins; these averages have been computed using the identical data sets as those that underly Fig. 3, see caption.